article_id
stringlengths 6
7
| url
stringlengths 19
20
| article_data
listlengths 1
7
| questions
listlengths 0
5
|
|---|---|---|---|
722661 | /viewarticle/722661 | [
{
"authors": "Syeda Sabahat Mansur, MB BS; Fardidullah Shah, MB BS",
"content": [
"Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.",
"A 45-year-old woman presents to the emergency department with bleeding gums and bruises on both forearms for the last 2 days. For the preceding 10 days she had been experiencing a high fever (which has since broken) and rigors. In addition, she complains of a rash over both forearms, but she is unable to further characterize it. She noted severe pain in both legs during the febrile portion of her illness. There was no history of hematuria, melena, cough, or hemoptysis. She is not taking any routine prescription medications or using over-the-counter products or supplements. She has no known drug allergies. She is married with 5 children and is currently unemployed. She does not smoke or drink alcohol and has no history of drug abuse. There is no travel history or any history of sick contacts. She is a resident of Pakistan."
],
"date": "November 24, 2014",
"figures": [],
"markdown": "# A 45-Year-Old Woman With Bleeding Gums and Bruising Following a Febrile Illness\n\n **Authors:** Syeda Sabahat Mansur, MB BS; Fardidullah Shah, MB BS \n **Date:** November 24, 2014\n\n ## Content\n\n Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.\nA 45-year-old woman presents to the emergency department with bleeding gums and bruises on both forearms for the last 2 days. For the preceding 10 days she had been experiencing a high fever (which has since broken) and rigors. In addition, she complains of a rash over both forearms, but she is unable to further characterize it. She noted severe pain in both legs during the febrile portion of her illness. There was no history of hematuria, melena, cough, or hemoptysis. She is not taking any routine prescription medications or using over-the-counter products or supplements. She has no known drug allergies. She is married with 5 children and is currently unemployed. She does not smoke or drink alcohol and has no history of drug abuse. There is no travel history or any history of sick contacts. She is a resident of Pakistan.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 45-Year-Old Woman With Bleeding Gums and Bruising Following a Febrile Illness"
},
{
"authors": "Syeda Sabahat Mansur, MB BS; Fardidullah Shah, MB BS",
"content": [
"On physical examination, she is alert and apparently well developed and well nourished. The patient has a regular pulse of 90 bpm and a respiratory rate of 14 breaths/min. Her temperature is 98.2° F (36.8° C) and blood pressure is 110/70 mm Hg. The cardiac examination reveals a normal S1 and S2, with no murmur, gallop, or rub. Auscultation of the lungs is normal, and no palpable organomegaly or tenderness is found on abdominal examination. Examination of the extremities reveals large bruises and a petechial rash across both forearms and lower extremities (Figure 1). Conjunctival hemorrhages are noted bilaterally. Bruises are also apparent on her soft palate, and minor trauma from oral examination results in gingival hemorrhage.",
"The laboratory investigation reveals a hemoglobin of 8 g/dL (80 g/L), platelet count of 11 × 103/µL (11 × 109/L), and a white blood cell count of 1.8 × 103/µL (1.8 × 109/L). Her serum blood urea nitrogen, creatinine, liver function tests, albumin, and electrolytes are normal. Coagulation studies, including a prothrombin time, activated partial thromboplastin time, fibrin degradation products, and serum fibrinogen are normal. Blood cultures do not show any growth. Urine analysis and urine culture result negative. Posteroanterior and lateral chest radiographs, as well as abdominal ultrasonography, are unrevealing.",
"Figure 1."
],
"date": "November 24, 2014",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/722/659/722659-thumb1.png"
}
],
"markdown": "# A 45-Year-Old Woman With Bleeding Gums and Bruising Following a Febrile Illness\n\n **Authors:** Syeda Sabahat Mansur, MB BS; Fardidullah Shah, MB BS \n **Date:** November 24, 2014\n\n ## Content\n\n On physical examination, she is alert and apparently well developed and well nourished. The patient has a regular pulse of 90 bpm and a respiratory rate of 14 breaths/min. Her temperature is 98.2° F (36.8° C) and blood pressure is 110/70 mm Hg. The cardiac examination reveals a normal S1 and S2, with no murmur, gallop, or rub. Auscultation of the lungs is normal, and no palpable organomegaly or tenderness is found on abdominal examination. Examination of the extremities reveals large bruises and a petechial rash across both forearms and lower extremities (Figure 1). Conjunctival hemorrhages are noted bilaterally. Bruises are also apparent on her soft palate, and minor trauma from oral examination results in gingival hemorrhage.\nThe laboratory investigation reveals a hemoglobin of 8 g/dL (80 g/L), platelet count of 11 × 103/µL (11 × 109/L), and a white blood cell count of 1.8 × 103/µL (1.8 × 109/L). Her serum blood urea nitrogen, creatinine, liver function tests, albumin, and electrolytes are normal. Coagulation studies, including a prothrombin time, activated partial thromboplastin time, fibrin degradation products, and serum fibrinogen are normal. Blood cultures do not show any growth. Urine analysis and urine culture result negative. Posteroanterior and lateral chest radiographs, as well as abdominal ultrasonography, are unrevealing.\nFigure 1.\n\n ## Figures\n\n **Figure 1.** \n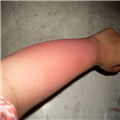 \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 345538,
"choiceText": "Leptospirosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345539,
"choiceText": "Meningococcemia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345540,
"choiceText": "<em>Plasmodium falciparum</em> malaria",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345541,
"choiceText": "Typhoid fever",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345542,
"choiceText": "Dengue hemorrhagic fever",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 97752,
"questionText": "Based on the clinical presentation and physical examination, which of the following is the most likely diagnosis?\r\n<br><br>\r\n<em>Hint: Bruises, conjunctival hemorrhages, and depressed cell lines in a postfebrile patient with a rash.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 45-Year-Old Woman With Bleeding Gums and Bruising Following a Febrile Illness"
},
{
"authors": "Syeda Sabahat Mansur, MB BS; Fardidullah Shah, MB BS",
"content": [
"This patient was diagnosed with dengue hemorrhagic fever (DHF), which is a complication of dengue fever (DF). The diagnosis was eventually confirmed by paired immunoglobulin M samples demonstrating an acute rise in antibodies.",
"Figure 1.",
"Dengue virus belongs to the family Flaviviridae (genus Flavivirus) and has emerged as the most common arboviral disease in the world. The disease is endemic to tropical and subtropical areas of the world, with about 2.5 billion people (40% of the world's population) at risk of acquiring the infection.[1] Dengue virus is transmitted to humans through the bites of infective female Aedes mosquitoes (particularly A aegypti and A albopictus). Mosquitoes generally acquire the virus while feeding on the blood of an infected person. After an incubation period of 8-10 days, an infected mosquito is capable, during probing and blood feeding, of transmitting the virus to susceptible individuals for the rest of its life.[2] Unlike malaria, which is more prevalent in rural areas, DF is spread via mosquitoes that thrive in highly populated urban environments.[3]"
],
"date": "November 24, 2014",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/722/659/722659-thumb1.png"
}
],
"markdown": "# A 45-Year-Old Woman With Bleeding Gums and Bruising Following a Febrile Illness\n\n **Authors:** Syeda Sabahat Mansur, MB BS; Fardidullah Shah, MB BS \n **Date:** November 24, 2014\n\n ## Content\n\n This patient was diagnosed with dengue hemorrhagic fever (DHF), which is a complication of dengue fever (DF). The diagnosis was eventually confirmed by paired immunoglobulin M samples demonstrating an acute rise in antibodies.\nFigure 1.\nDengue virus belongs to the family Flaviviridae (genus Flavivirus) and has emerged as the most common arboviral disease in the world. The disease is endemic to tropical and subtropical areas of the world, with about 2.5 billion people (40% of the world's population) at risk of acquiring the infection.[1] Dengue virus is transmitted to humans through the bites of infective female Aedes mosquitoes (particularly A aegypti and A albopictus). Mosquitoes generally acquire the virus while feeding on the blood of an infected person. After an incubation period of 8-10 days, an infected mosquito is capable, during probing and blood feeding, of transmitting the virus to susceptible individuals for the rest of its life.[2] Unlike malaria, which is more prevalent in rural areas, DF is spread via mosquitoes that thrive in highly populated urban environments.[3]\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 345538,
"choiceText": "Leptospirosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345539,
"choiceText": "Meningococcemia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345540,
"choiceText": "<em>Plasmodium falciparum</em> malaria",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345541,
"choiceText": "Typhoid fever",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345542,
"choiceText": "Dengue hemorrhagic fever",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 97752,
"questionText": "Based on the clinical presentation and physical examination, which of the following is the most likely diagnosis?\r\n<br><br>\r\n<em>Hint: Bruises, conjunctival hemorrhages, and depressed cell lines in a postfebrile patient with a rash.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 45-Year-Old Woman With Bleeding Gums and Bruising Following a Febrile Illness"
},
{
"authors": "Syeda Sabahat Mansur, MB BS; Fardidullah Shah, MB BS",
"content": [
"Four distinct, but closely related, viruses (termed dengue virus types 1-4 [DENV 1-4]) cause DF. Humans are the main amplifying host of the virus.[4] Infection with 1 of the 4 serotypes of dengue virus causes a wide spectrum of clinical disease, including asymptomatic infection, undifferentiated fever, DF, and DHF. DHF occurs in a minority of patients and is characterized by bleeding and plasma leakage, which may lead to shock.[5] The major risk factor for DHF is prior immunity to a single dengue virus serotype. Infection with one dengue serotype confers lifelong homotypic immunity and a very brief period of partial heterotypic immunity (approximately 6 mo), but an individual can eventually be infected by more than one serotype. An individual could therefore experience a case of DENV-1 fever in one year, followed by a case of DENV-2 fever in the following year. Third infections are, however, very rare, and fourth infections have never been reported.[6] Several serotypes can be in circulation during a particular epidemic.[7]",
"Some people infected with DF are asymptomatic. Young children often have a fever with a rash, but other symptoms are minor. Older children and adults may also have mild symptoms; however, they are more likely to experience classic DF.[2] Symptoms of DF include a high fever (up to 105° F [40.5° C]), severe headache, retro-orbital pain, severe muscle and joint pain, swollen lymph nodes, general malaise, nausea, and vomiting; a macular erythematous rash with petechiae may also be observed.[7] The differential diagnosis for DF and DHF is broad and includes meningococcal meningitis, septicemia and disseminated intravascular coagulation, other hemorrhagic fevers (eg, Crimean Congo hemorrhagic fever, Ebola), thrombotic thrombocytopenic purpura, falciparum malaria, leptospirosis, aplastic anemia, acute leukemia, and yellow fever.",
"Direct person-to-person transmission of Dengue virus has not been documented. A few case reports have been published of transmission of DENV through exposure to dengue-infected blood, organs, or other tissues from blood transfusions; solid organ or bone marrow transplants; needle stick injuries; and mucous membrane contact with dengue-infected blood.[8]",
"Dengue or dengue like epidemics were reported throughout the nineteenth and early twentieth centuries in America, Southern Europe, North Africa, the east Mediterranean, Asia, Australia, and on various islands in the Indian Ocean, the south and Central Pacific, and the Caribbean. DHF has increased both in incidence and distribution over the past 40 years, and, in 1996, 2.5-3 billion people lived in areas potentially at risk for dengue virus transmission. It is estimated that there are 20 million cases of dengue infection annually, resulting in around 24,000 deaths.[9] The geographic distribution of dengue viruses and their mosquito vectors has expanded, and DHF has emerged in the Pacific region and the Americas. In Southeast Asia, epidemic DHF first appeared in the 1950s, but by 1975 it had become a leading cause of hospitalization and death among children in many countries in that region.[10] In Europe, the last dengue epidemic dates from 1927-1928 in Greece, with high mortality. However, cases of DF are still reported in travelers returning to Europe from endemic areas.[11]",
"In the 1980s, DHF began a second expansion into Asia when Sri Lanka, India, and the Maldives Islands had their first major DHF epidemics; Pakistan first reported an epidemic of DF in 1994. The epidemics in Sri Lanka and India were associated with multiple dengue virus serotypes. After an absence of 35 years, epidemic DF occurred in both Taiwan and the People's Republic of China in the 1980s. The People's Republic of China had a series of epidemics caused by all 4 serotypes, and its first major epidemic of DHF, caused by DENV-2, was reported on Hainan Island in 1985. Singapore also had a resurgence of DF/DHF from 1990 to 1994 after a successful control program had prevented significant transmission for over 20 years. In other countries in Asia where DHF is endemic, the epidemics have become progressively larger in the last 15 years.[10]",
"An outbreak of DF in Karachi occurred in 2005 when Aga Khan University reported 30 positive cases out of 100. A recent trend of DF in southeastern countries is that it has become endemic, causing cyclical epidemics every 2-3 years.[12]"
],
"date": "November 24, 2014",
"figures": [],
"markdown": "# A 45-Year-Old Woman With Bleeding Gums and Bruising Following a Febrile Illness\n\n **Authors:** Syeda Sabahat Mansur, MB BS; Fardidullah Shah, MB BS \n **Date:** November 24, 2014\n\n ## Content\n\n Four distinct, but closely related, viruses (termed dengue virus types 1-4 [DENV 1-4]) cause DF. Humans are the main amplifying host of the virus.[4] Infection with 1 of the 4 serotypes of dengue virus causes a wide spectrum of clinical disease, including asymptomatic infection, undifferentiated fever, DF, and DHF. DHF occurs in a minority of patients and is characterized by bleeding and plasma leakage, which may lead to shock.[5] The major risk factor for DHF is prior immunity to a single dengue virus serotype. Infection with one dengue serotype confers lifelong homotypic immunity and a very brief period of partial heterotypic immunity (approximately 6 mo), but an individual can eventually be infected by more than one serotype. An individual could therefore experience a case of DENV-1 fever in one year, followed by a case of DENV-2 fever in the following year. Third infections are, however, very rare, and fourth infections have never been reported.[6] Several serotypes can be in circulation during a particular epidemic.[7]\nSome people infected with DF are asymptomatic. Young children often have a fever with a rash, but other symptoms are minor. Older children and adults may also have mild symptoms; however, they are more likely to experience classic DF.[2] Symptoms of DF include a high fever (up to 105° F [40.5° C]), severe headache, retro-orbital pain, severe muscle and joint pain, swollen lymph nodes, general malaise, nausea, and vomiting; a macular erythematous rash with petechiae may also be observed.[7] The differential diagnosis for DF and DHF is broad and includes meningococcal meningitis, septicemia and disseminated intravascular coagulation, other hemorrhagic fevers (eg, Crimean Congo hemorrhagic fever, Ebola), thrombotic thrombocytopenic purpura, falciparum malaria, leptospirosis, aplastic anemia, acute leukemia, and yellow fever.\nDirect person-to-person transmission of Dengue virus has not been documented. A few case reports have been published of transmission of DENV through exposure to dengue-infected blood, organs, or other tissues from blood transfusions; solid organ or bone marrow transplants; needle stick injuries; and mucous membrane contact with dengue-infected blood.[8]\nDengue or dengue like epidemics were reported throughout the nineteenth and early twentieth centuries in America, Southern Europe, North Africa, the east Mediterranean, Asia, Australia, and on various islands in the Indian Ocean, the south and Central Pacific, and the Caribbean. DHF has increased both in incidence and distribution over the past 40 years, and, in 1996, 2.5-3 billion people lived in areas potentially at risk for dengue virus transmission. It is estimated that there are 20 million cases of dengue infection annually, resulting in around 24,000 deaths.[9] The geographic distribution of dengue viruses and their mosquito vectors has expanded, and DHF has emerged in the Pacific region and the Americas. In Southeast Asia, epidemic DHF first appeared in the 1950s, but by 1975 it had become a leading cause of hospitalization and death among children in many countries in that region.[10] In Europe, the last dengue epidemic dates from 1927-1928 in Greece, with high mortality. However, cases of DF are still reported in travelers returning to Europe from endemic areas.[11]\nIn the 1980s, DHF began a second expansion into Asia when Sri Lanka, India, and the Maldives Islands had their first major DHF epidemics; Pakistan first reported an epidemic of DF in 1994. The epidemics in Sri Lanka and India were associated with multiple dengue virus serotypes. After an absence of 35 years, epidemic DF occurred in both Taiwan and the People's Republic of China in the 1980s. The People's Republic of China had a series of epidemics caused by all 4 serotypes, and its first major epidemic of DHF, caused by DENV-2, was reported on Hainan Island in 1985. Singapore also had a resurgence of DF/DHF from 1990 to 1994 after a successful control program had prevented significant transmission for over 20 years. In other countries in Asia where DHF is endemic, the epidemics have become progressively larger in the last 15 years.[10]\nAn outbreak of DF in Karachi occurred in 2005 when Aga Khan University reported 30 positive cases out of 100. A recent trend of DF in southeastern countries is that it has become endemic, causing cyclical epidemics every 2-3 years.[12]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 45-Year-Old Woman With Bleeding Gums and Bruising Following a Febrile Illness"
},
{
"authors": "Syeda Sabahat Mansur, MB BS; Fardidullah Shah, MB BS",
"content": [
"A major challenge for public health officials in all tropical areas of the world is the development and implementation of sustainable prevention and control programs that will reverse the trend of emergent DHF.[13] Environmental controls, including solid waste management, decreasing vector breeding sites by eliminating standing water, improvement in public awareness by media, and the use of household insecticides and mosquito repellants can help prevent the spread of dengue virus. Active case surveillance is important for early detection and implementation of control programs in the setting of acute epidemics.[11] Unfortunately, there is no commercially available vaccine to prevent dengue.[14] Tetravalent vaccines are currently being studied.",
"Clinically, the diagnosis of DF is suggested by the presence of fever, severe headache, maculopapular skin rash, and myalgias associated with either the isolation or identification of DENV from either serum, plasma, or tissue specimens, or by demonstration of a 4-fold increase of DENV antibodies in paired serum samples. The diagnosis of DHF is based on similar clinical features associated with a bleeding diathesis and/or thrombocytopenia. In some patients, a shock syndrome (dengue shock syndrome) may be observed.",
"The treatment of DF and DHF is essentially supportive. Antipyretics as well as fluid resuscitation, monitoring, and support are often necessary. Monitoring of laboratory parameters and replenishment with blood products are necessary as indicated in severe cases of DHF. The World Health Organization has created a useful guide (Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control; available at the WHO Website[9]) that delineates recommended approaches to the identification and management of DHF.",
"The patient presented in this case was admitted to an inpatient medical ward for 10 days and managed with intravenous fluids as well as repeated platelet and packed red blood cell transfusions. She was discharged when her platelet count reached 60 × 103/µL (60 × 109/L). She returned to the outpatient department after 3 weeks for follow-up, at which time her bleeding, rash, and other symptoms had improved."
],
"date": "November 24, 2014",
"figures": [],
"markdown": "# A 45-Year-Old Woman With Bleeding Gums and Bruising Following a Febrile Illness\n\n **Authors:** Syeda Sabahat Mansur, MB BS; Fardidullah Shah, MB BS \n **Date:** November 24, 2014\n\n ## Content\n\n A major challenge for public health officials in all tropical areas of the world is the development and implementation of sustainable prevention and control programs that will reverse the trend of emergent DHF.[13] Environmental controls, including solid waste management, decreasing vector breeding sites by eliminating standing water, improvement in public awareness by media, and the use of household insecticides and mosquito repellants can help prevent the spread of dengue virus. Active case surveillance is important for early detection and implementation of control programs in the setting of acute epidemics.[11] Unfortunately, there is no commercially available vaccine to prevent dengue.[14] Tetravalent vaccines are currently being studied.\nClinically, the diagnosis of DF is suggested by the presence of fever, severe headache, maculopapular skin rash, and myalgias associated with either the isolation or identification of DENV from either serum, plasma, or tissue specimens, or by demonstration of a 4-fold increase of DENV antibodies in paired serum samples. The diagnosis of DHF is based on similar clinical features associated with a bleeding diathesis and/or thrombocytopenia. In some patients, a shock syndrome (dengue shock syndrome) may be observed.\nThe treatment of DF and DHF is essentially supportive. Antipyretics as well as fluid resuscitation, monitoring, and support are often necessary. Monitoring of laboratory parameters and replenishment with blood products are necessary as indicated in severe cases of DHF. The World Health Organization has created a useful guide (Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control; available at the WHO Website[9]) that delineates recommended approaches to the identification and management of DHF.\nThe patient presented in this case was admitted to an inpatient medical ward for 10 days and managed with intravenous fluids as well as repeated platelet and packed red blood cell transfusions. She was discharged when her platelet count reached 60 × 103/µL (60 × 109/L). She returned to the outpatient department after 3 weeks for follow-up, at which time her bleeding, rash, and other symptoms had improved.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 345543,
"choiceText": "The patient is infected with DENV-2",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345544,
"choiceText": "The patient is experiencing his second dengue infection with a different serotype",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345545,
"choiceText": "The patient has been previously infected with DENV-1, and there is a current epidemic of DENV-1 in his regional area",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345546,
"choiceText": "The patient has just returned from an area with a current Ebola outbreak",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Dengue fever is an acute, mosquito-transmitted viral disease caused by any 1 of 4 virus serotypes (DENV-1-4). Infection with any of these causes a wide spectrum of clinical disease, ranging from asymptomatic infection, undifferentiated fever, and DF to DHF. Infection with one dengue serotype confers lifelong homotypic immunity and a very brief period of partial heterotypic immunity, but each individual can eventually be infected by more than one serotype. It is thought that subsequent infections with different serotypes in individuals put patients at risk for more severe manifestations of disease, including DHF. This is thought to be due to partial immunity, which may cause an amplification rather than a mediation of illness. Ebola is one of the many diseases in the differential diagnosis of dengue fever. Visiting an area with an active Ebola outbreak should raise suspicion for Ebola.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 97753,
"questionText": "You are examining 30-year-old man who presents with increased bruising following a short bout of febrile illness accompanied by severe headaches, a rash, and muscle pain. You suspect DHF. Which of the following situations puts this patient at greatest risk of developing DHF?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 345547,
"choiceText": "Initiate immediate broad-spectrum antibiotics and early goal directed therapy (EGDT)",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345548,
"choiceText": "Plasmapheresis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345549,
"choiceText": "Oral acyclovir",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345550,
"choiceText": "Supportive care with fluid resuscitation, antipyretics, and blood product replacement as needed",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The treatment of DF and DHF is essentially supportive. Antipyretics as well as fluid resuscitation, monitoring, and support are often necessary. Monitoring of laboratory parameters and replenishment with blood products are indicated as necessary in severe cases of DHF. Antibiotics and EGDT are indicated for patients with significant bacterial infections and should likely be started empirically for severely ill, undifferentiated patients. However, they do not have a role in confirmed DHF. Plasmapheresis is indicated for treating thrombotic thrombocytopenic purpura, an illness in the differential diagnosis of dengue fever. Acyclovir is a treatment for herpes virus but is not indicated for DF.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 97754,
"questionText": "The diagnosis of DHF is confirmed with paired immunoglobulin M samples in the patient above. What is the most appropriate treatment plan for this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 45-Year-Old Woman With Bleeding Gums and Bruising Following a Febrile Illness"
},
{
"authors": "Syeda Sabahat Mansur, MB BS; Fardidullah Shah, MB BS",
"content": [],
"date": "November 24, 2014",
"figures": [],
"markdown": "# A 45-Year-Old Woman With Bleeding Gums and Bruising Following a Febrile Illness\n\n **Authors:** Syeda Sabahat Mansur, MB BS; Fardidullah Shah, MB BS \n **Date:** November 24, 2014\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 345543,
"choiceText": "The patient is infected with DENV-2",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345544,
"choiceText": "The patient is experiencing his second dengue infection with a different serotype",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345545,
"choiceText": "The patient has been previously infected with DENV-1, and there is a current epidemic of DENV-1 in his regional area",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345546,
"choiceText": "The patient has just returned from an area with a current Ebola outbreak",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Dengue fever is an acute, mosquito-transmitted viral disease caused by any 1 of 4 virus serotypes (DENV-1-4). Infection with any of these causes a wide spectrum of clinical disease, ranging from asymptomatic infection, undifferentiated fever, and DF to DHF. Infection with one dengue serotype confers lifelong homotypic immunity and a very brief period of partial heterotypic immunity, but each individual can eventually be infected by more than one serotype. It is thought that subsequent infections with different serotypes in individuals put patients at risk for more severe manifestations of disease, including DHF. This is thought to be due to partial immunity, which may cause an amplification rather than a mediation of illness. Ebola is one of the many diseases in the differential diagnosis of dengue fever. Visiting an area with an active Ebola outbreak should raise suspicion for Ebola.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 97753,
"questionText": "You are examining 30-year-old man who presents with increased bruising following a short bout of febrile illness accompanied by severe headaches, a rash, and muscle pain. You suspect DHF. Which of the following situations puts this patient at greatest risk of developing DHF?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 345547,
"choiceText": "Initiate immediate broad-spectrum antibiotics and early goal directed therapy (EGDT)",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345548,
"choiceText": "Plasmapheresis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345549,
"choiceText": "Oral acyclovir",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345550,
"choiceText": "Supportive care with fluid resuscitation, antipyretics, and blood product replacement as needed",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The treatment of DF and DHF is essentially supportive. Antipyretics as well as fluid resuscitation, monitoring, and support are often necessary. Monitoring of laboratory parameters and replenishment with blood products are indicated as necessary in severe cases of DHF. Antibiotics and EGDT are indicated for patients with significant bacterial infections and should likely be started empirically for severely ill, undifferentiated patients. However, they do not have a role in confirmed DHF. Plasmapheresis is indicated for treating thrombotic thrombocytopenic purpura, an illness in the differential diagnosis of dengue fever. Acyclovir is a treatment for herpes virus but is not indicated for DF.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 97754,
"questionText": "The diagnosis of DHF is confirmed with paired immunoglobulin M samples in the patient above. What is the most appropriate treatment plan for this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 45-Year-Old Woman With Bleeding Gums and Bruising Following a Febrile Illness"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 345538,
"choiceText": "Leptospirosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345539,
"choiceText": "Meningococcemia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345540,
"choiceText": "<em>Plasmodium falciparum</em> malaria",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345541,
"choiceText": "Typhoid fever",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345542,
"choiceText": "Dengue hemorrhagic fever",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 97752,
"questionText": "Based on the clinical presentation and physical examination, which of the following is the most likely diagnosis?\r\n<br><br>\r\n<em>Hint: Bruises, conjunctival hemorrhages, and depressed cell lines in a postfebrile patient with a rash.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 345543,
"choiceText": "The patient is infected with DENV-2",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345544,
"choiceText": "The patient is experiencing his second dengue infection with a different serotype",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345545,
"choiceText": "The patient has been previously infected with DENV-1, and there is a current epidemic of DENV-1 in his regional area",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345546,
"choiceText": "The patient has just returned from an area with a current Ebola outbreak",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Dengue fever is an acute, mosquito-transmitted viral disease caused by any 1 of 4 virus serotypes (DENV-1-4). Infection with any of these causes a wide spectrum of clinical disease, ranging from asymptomatic infection, undifferentiated fever, and DF to DHF. Infection with one dengue serotype confers lifelong homotypic immunity and a very brief period of partial heterotypic immunity, but each individual can eventually be infected by more than one serotype. It is thought that subsequent infections with different serotypes in individuals put patients at risk for more severe manifestations of disease, including DHF. This is thought to be due to partial immunity, which may cause an amplification rather than a mediation of illness. Ebola is one of the many diseases in the differential diagnosis of dengue fever. Visiting an area with an active Ebola outbreak should raise suspicion for Ebola.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 97753,
"questionText": "You are examining 30-year-old man who presents with increased bruising following a short bout of febrile illness accompanied by severe headaches, a rash, and muscle pain. You suspect DHF. Which of the following situations puts this patient at greatest risk of developing DHF?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 345547,
"choiceText": "Initiate immediate broad-spectrum antibiotics and early goal directed therapy (EGDT)",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345548,
"choiceText": "Plasmapheresis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345549,
"choiceText": "Oral acyclovir",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 345550,
"choiceText": "Supportive care with fluid resuscitation, antipyretics, and blood product replacement as needed",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The treatment of DF and DHF is essentially supportive. Antipyretics as well as fluid resuscitation, monitoring, and support are often necessary. Monitoring of laboratory parameters and replenishment with blood products are indicated as necessary in severe cases of DHF. Antibiotics and EGDT are indicated for patients with significant bacterial infections and should likely be started empirically for severely ill, undifferentiated patients. However, they do not have a role in confirmed DHF. Plasmapheresis is indicated for treating thrombotic thrombocytopenic purpura, an illness in the differential diagnosis of dengue fever. Acyclovir is a treatment for herpes virus but is not indicated for DF.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 97754,
"questionText": "The diagnosis of DHF is confirmed with paired immunoglobulin M samples in the patient above. What is the most appropriate treatment plan for this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
721812 | /viewarticle/721812 | [
{
"authors": "Christine A. Ebert-Santos, MD; Anicia Santos",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 3-month-old male infant is presented to the pediatric clinic for a routine well-child examination and care. The birth and perinatal history include a spontaneous vaginal delivery at term to a G3 P2 31-year-old woman with normal prenatal sonograms and excellent prenatal care. The prenatal history was uneventful.",
"Upon circumcision at age 4 days, he experienced a choking spell after being restrained, but he recovered without further incident. He has been breastfeeding since being discharged from the hospital, and at that time he weighed 6 lb 7 oz. Upon follow-up, he had normal oxygen saturation and his weight was up to 6 lb 12 oz at his 2-week examination. No significant family history is reported. He is not currently on any medications and has no history of any known allergies."
],
"date": "July 17, 2017",
"figures": [],
"markdown": "# A 3-Month-Old Boy With Severe Failure to Thrive and Hypercalcemia\n\n **Authors:** Christine A. Ebert-Santos, MD; Anicia Santos \n **Date:** July 17, 2017\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 3-month-old male infant is presented to the pediatric clinic for a routine well-child examination and care. The birth and perinatal history include a spontaneous vaginal delivery at term to a G3 P2 31-year-old woman with normal prenatal sonograms and excellent prenatal care. The prenatal history was uneventful.\nUpon circumcision at age 4 days, he experienced a choking spell after being restrained, but he recovered without further incident. He has been breastfeeding since being discharged from the hospital, and at that time he weighed 6 lb 7 oz. Upon follow-up, he had normal oxygen saturation and his weight was up to 6 lb 12 oz at his 2-week examination. No significant family history is reported. He is not currently on any medications and has no history of any known allergies.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 3-Month-Old Boy With Severe Failure to Thrive and Hypercalcemia"
},
{
"authors": "Christine A. Ebert-Santos, MD; Anicia Santos",
"content": [
"On examination, the baby, although smiling, appears to be severely malnourished. He has an absence of subcutaneous fat, and his skin appears wrinkled and tented. He does not seem to be in any acute distress. His vital signs include a pulse of 130 bpm, respiratory rate of 28 breaths/min, temperature of 98.2°F (36.8°C), and blood pressure of 127/71 mm Hg. His weight is 6 lb 5 oz, which is below the 3rd percentile for an infant of this age, and he is measured at 21 in long, which also falls below the 3rd percentile for his age. His head circumference is 14.75 in (also below the 3rd percentile). He weighs less now than he did at birth. His abdomen appears bloated, but no evidence of any organomegaly is present.",
"The heart and lung examinations findings are normal. Further examination reveals apparently normal hearing and sight. According to the parents, the baby only takes about 7 oz of baby formula per day (approximately 0.5-1 oz of formula every 2-3 hours and 0.5-1 oz of breast milk per day). The patient cries when feeding in the office. The child's mother has switched him to a low-flow nipple as a precaution against choking.",
"The patient is admitted for 10 days in the hospital. When examined afterwards, the patient is found to still have no appetite, continues to cry when feeding (although his weight has increased to 8 lb), and appears more hypoxic than normal babies.",
"Laboratory tests done at the time of hospitalization show that the patient has severe hypercalcemia, with a total calcium of 4.53 mmol/L (normal range, 2.25-2.80 mmol/L) and an ionized calcium of 8.8 mg/dL. He also has a low normal phosphate of 4.4-4.5 mg/dL. The alkaline phosphatase level is 93 mg/dL (normal range, 143-320 mg/dL). His urine phosphoethanolamine level is 2142 nmol/mg of creatinine, and his vitamin B6 level is >100 µg/dL. 2-dimensional echocardiography findings are normal; however, a radiographic inspection of his bone quality reveals irregular metaphyses, along with severe metaphyseal flaring and osteopenia (see Figures 1-3).",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"Renal ultrasonography reveals bilateral hydronephrosis that is moderate on the left and mild on the right. An obstruction at the uretero-pelvic junction on the left is possible, with a milder obstruction on the right. A voiding cystourethrogram (VCUG) is negative. The patient's oxygen saturation is 72%-76% on room air; however, this measurement is obtained at his home, which is at an elevation of 10,000 feet."
],
"date": "July 17, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/721/812/721812-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/721/812/721812-thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/721/812/721812-thumb3.png"
}
],
"markdown": "# A 3-Month-Old Boy With Severe Failure to Thrive and Hypercalcemia\n\n **Authors:** Christine A. Ebert-Santos, MD; Anicia Santos \n **Date:** July 17, 2017\n\n ## Content\n\n On examination, the baby, although smiling, appears to be severely malnourished. He has an absence of subcutaneous fat, and his skin appears wrinkled and tented. He does not seem to be in any acute distress. His vital signs include a pulse of 130 bpm, respiratory rate of 28 breaths/min, temperature of 98.2°F (36.8°C), and blood pressure of 127/71 mm Hg. His weight is 6 lb 5 oz, which is below the 3rd percentile for an infant of this age, and he is measured at 21 in long, which also falls below the 3rd percentile for his age. His head circumference is 14.75 in (also below the 3rd percentile). He weighs less now than he did at birth. His abdomen appears bloated, but no evidence of any organomegaly is present.\nThe heart and lung examinations findings are normal. Further examination reveals apparently normal hearing and sight. According to the parents, the baby only takes about 7 oz of baby formula per day (approximately 0.5-1 oz of formula every 2-3 hours and 0.5-1 oz of breast milk per day). The patient cries when feeding in the office. The child's mother has switched him to a low-flow nipple as a precaution against choking.\nThe patient is admitted for 10 days in the hospital. When examined afterwards, the patient is found to still have no appetite, continues to cry when feeding (although his weight has increased to 8 lb), and appears more hypoxic than normal babies.\nLaboratory tests done at the time of hospitalization show that the patient has severe hypercalcemia, with a total calcium of 4.53 mmol/L (normal range, 2.25-2.80 mmol/L) and an ionized calcium of 8.8 mg/dL. He also has a low normal phosphate of 4.4-4.5 mg/dL. The alkaline phosphatase level is 93 mg/dL (normal range, 143-320 mg/dL). His urine phosphoethanolamine level is 2142 nmol/mg of creatinine, and his vitamin B6 level is >100 µg/dL. 2-dimensional echocardiography findings are normal; however, a radiographic inspection of his bone quality reveals irregular metaphyses, along with severe metaphyseal flaring and osteopenia (see Figures 1-3).\nFigure 1.\nFigure 2.\nFigure 3.\nRenal ultrasonography reveals bilateral hydronephrosis that is moderate on the left and mild on the right. An obstruction at the uretero-pelvic junction on the left is possible, with a milder obstruction on the right. A voiding cystourethrogram (VCUG) is negative. The patient's oxygen saturation is 72%-76% on room air; however, this measurement is obtained at his home, which is at an elevation of 10,000 feet.\n\n ## Figures\n\n **Figure 1.** \n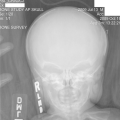 \n\n**Figure 2.** \n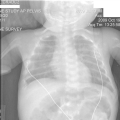 \n\n**Figure 3.** \n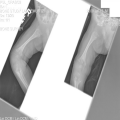 \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 342999,
"choiceText": "Osteogenesis imperfecta",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 343000,
"choiceText": "Hypophosphatasia",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 343001,
"choiceText": "Bartter syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 343002,
"choiceText": "Vitamin D deficiency (rickets)",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 96836,
"questionText": "Which of the following is the most likely diagnosis? \r\n<br /><br /><em>Hint: Keep in mind the severe hypercalcemia, alkaline phosphatase, and radiology results.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 3-Month-Old Boy With Severe Failure to Thrive and Hypercalcemia"
},
{
"authors": "Christine A. Ebert-Santos, MD; Anicia Santos",
"content": [
"A diagnosis of hypophosphatasia, also known as Rathbun syndrome, was made on the basis of the radiologic findings and the results of the blood chemistry tests. Initially, several diagnoses were possible; all of the ones listed in the answer choices above were considered at some point. The typical findings of metaphyseal flaring and osteopenia, however, pointed to infantile hypophosphatasia. The elevated phosphoethanolamine levels and below-normal alkaline phosphatase levels also supported the diagnosis.",
"Phosphoethanolamine levels in urine are usually between 180 and 533 nmol/mg of creatinine. Normal alkaline phosphatase levels are between 143 and 320 mg/dL. The serum calcium level of 4.53 mmol/L was well above the normal range of 2.25-2.80 mmol/L. The levels of vitamin B6 were also extremely elevated, the normal range being between 5.3 and 46.7 μg/dL. Other patients with hypophosphatasia have also presented with what appeared to be an obstruction at the level of the ureteropelvic junction, although this is not typical in all cases.",
"Severe hypophosphatasia occurs in approximately 1 in every 100,000 live births in the United States. It is an autosomal-recessive disorder that is believed to be caused by a molecular defect in the gene encoding tissue-nonspecific alkaline phosphatase (TNSALP).[1] TNSALP is an ectoenzyme tethered to the outer surface of osteoblast and chondrocyte cell membranes. TNSALP normally hydrolyzes several substances, including inorganic pyrophosphate (PPi) and pyridoxal 5'-phosphate (PLP), a major form of vitamin B6. The less severe forms that appear later in life may either be autosomal-recessive or -dominant disorders. The main cause of abnormal bone mineralization is a result of the deficiency of alkaline phosphatase in the bone and liver. The mechanism is not yet fully understood. Alkaline phosphatase in the intestinal, placental, and germ cells appear to be unaffected.[1]"
],
"date": "July 17, 2017",
"figures": [],
"markdown": "# A 3-Month-Old Boy With Severe Failure to Thrive and Hypercalcemia\n\n **Authors:** Christine A. Ebert-Santos, MD; Anicia Santos \n **Date:** July 17, 2017\n\n ## Content\n\n A diagnosis of hypophosphatasia, also known as Rathbun syndrome, was made on the basis of the radiologic findings and the results of the blood chemistry tests. Initially, several diagnoses were possible; all of the ones listed in the answer choices above were considered at some point. The typical findings of metaphyseal flaring and osteopenia, however, pointed to infantile hypophosphatasia. The elevated phosphoethanolamine levels and below-normal alkaline phosphatase levels also supported the diagnosis.\nPhosphoethanolamine levels in urine are usually between 180 and 533 nmol/mg of creatinine. Normal alkaline phosphatase levels are between 143 and 320 mg/dL. The serum calcium level of 4.53 mmol/L was well above the normal range of 2.25-2.80 mmol/L. The levels of vitamin B6 were also extremely elevated, the normal range being between 5.3 and 46.7 μg/dL. Other patients with hypophosphatasia have also presented with what appeared to be an obstruction at the level of the ureteropelvic junction, although this is not typical in all cases.\nSevere hypophosphatasia occurs in approximately 1 in every 100,000 live births in the United States. It is an autosomal-recessive disorder that is believed to be caused by a molecular defect in the gene encoding tissue-nonspecific alkaline phosphatase (TNSALP).[1] TNSALP is an ectoenzyme tethered to the outer surface of osteoblast and chondrocyte cell membranes. TNSALP normally hydrolyzes several substances, including inorganic pyrophosphate (PPi) and pyridoxal 5'-phosphate (PLP), a major form of vitamin B6. The less severe forms that appear later in life may either be autosomal-recessive or -dominant disorders. The main cause of abnormal bone mineralization is a result of the deficiency of alkaline phosphatase in the bone and liver. The mechanism is not yet fully understood. Alkaline phosphatase in the intestinal, placental, and germ cells appear to be unaffected.[1]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 342999,
"choiceText": "Osteogenesis imperfecta",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 343000,
"choiceText": "Hypophosphatasia",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 343001,
"choiceText": "Bartter syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 343002,
"choiceText": "Vitamin D deficiency (rickets)",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 96836,
"questionText": "Which of the following is the most likely diagnosis? \r\n<br /><br /><em>Hint: Keep in mind the severe hypercalcemia, alkaline phosphatase, and radiology results.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 3-Month-Old Boy With Severe Failure to Thrive and Hypercalcemia"
},
{
"authors": "Christine A. Ebert-Santos, MD; Anicia Santos",
"content": [
"Six different forms of hypophosphatasia are recognized. They include perinatal, infantile, childhood, adult biphasic, adult monophasic, and odontohypophosphatasia. Ultrasonography can be used to successfully identify the disorder in a fetus, and the diagnosis can be confirmed with genetic testing. The most severe perinatal hypophosphatasia may be identified when a stillborn child is found to have no mineralized bone. However, the least severe adult form may present simply as a pathologic fracture in an adult.[2] A combination of biochemical testing for hypercalcemia, decreased levels of alkaline phosphatase, and radiography to detect findings such as metaphyseal flaring, abnormally large fontanelle, and abnormally wide sutures can be used to diagnose infantile hypophosphatasia. In the less severe forms, or those of later onset, often the only sign is the loss of deciduous teeth.",
"Patients with infantile hypophosphatasia, such as the patient described in this case study, may show signs at birth or the signs and symptoms may manifest within the first 6 months of life. Respiratory complications are typical due to a rachitic chest, a finding which may lead to the misdiagnosis of vitamin D deficiency (rickets), especially when coupled with some of the findings on blood chemistry. Hypercalcemia is also typical of patients with hypophosphatasia. Infantile hypophosphatasia usually presents with early failure to thrive, hypotonia, seizures, irritability, anemia, hypercalciuria, and phosphoethanolaminuria. Patients may exhibit blue sclerae, bowed short limbs, metaphyseal cupping, bony spurs on the ulna and fibula, lack of skeletal ossification, easy fracturing, craniosynostosis, and poorly formed teeth.[3]Hypercalcemia and hypercalciuria can cause vomiting and compromise the kidneys. Nephrocalcinosis and seizures may also be present in a patient affected with infantile hypophosphatasia.[4]",
"Childhood hypophosphatasia is usually diagnosed by a dentist when premature loss of deciduous teeth is seen. As stated, this may be the only symptom, and other symptoms may be ascribed to other bone diseases. Those affected may also have stunted growth, a waddling gait, and learn to walk later than normal.",
"Adult hypophosphatasia presents in 2 forms: a biphasic type, which appears during childhood and is often not diagnosed until it becomes much more active during middle age; and a monophasic form, which appears later in life with milder symptoms. Both of these forms are generally not diagnosed until the fourth decade of life. Tooth loss, metatarsal stress fractures, and femur fractures, which cause hip and thigh pain in adults are indicative of this diagnosis.",
"Odontohypophosphatasia presents only as through the loss of deciduous teeth and does not appear with any other symptoms of decreased mineralization.[4]",
"Overall, hypophosphatasia is considered quite similar to osteogenesis imperfecta. Many of the symptoms are common to both diseases, such as the blue sclera and the pathologic fractures. Unlike hypophosphatasia, however, osteogenesis imperfecta does not correlate with a decreased alkaline phosphatase level concomitant with an increase in other endogenous substances, such as vitamin B6 and phosphoethanolamine. This patient's endocrinologist also suggested hypervitaminosis D, subcutaneous fat necrosis, Bartter syndrome, Williams syndrome, Jansen syndrome, and embryonal renal tumor as possible causes for the patient's symptoms.",
"Hypervitaminosis D was ruled out because it was not supported by any of the laboratory findings. Subcutaneous fat necrosis did not account for the radiologic findings. None of the other diagnoses could account for the combination of symptoms seen in this 3-month-old. Hypophosphatasia was indicated clinically, biochemically, and radiologically. The severe failure to thrive, blue sclera, and flaring at the wrists are all typical of the condition. Laboratory analysis showed the expected decreased alkaline phosphatase activity, elevated urinary phosphoethanolamine levels, and high serum levels of vitamin B6. In addition, a fluorescent in situ hybridization (FISH) test for the Williams syndrome deletion was negative. The radiology report that showed metaphyseal flaring and osteopenia further pointed towards a diagnosis of hypophosphatasia."
],
"date": "July 17, 2017",
"figures": [],
"markdown": "# A 3-Month-Old Boy With Severe Failure to Thrive and Hypercalcemia\n\n **Authors:** Christine A. Ebert-Santos, MD; Anicia Santos \n **Date:** July 17, 2017\n\n ## Content\n\n Six different forms of hypophosphatasia are recognized. They include perinatal, infantile, childhood, adult biphasic, adult monophasic, and odontohypophosphatasia. Ultrasonography can be used to successfully identify the disorder in a fetus, and the diagnosis can be confirmed with genetic testing. The most severe perinatal hypophosphatasia may be identified when a stillborn child is found to have no mineralized bone. However, the least severe adult form may present simply as a pathologic fracture in an adult.[2] A combination of biochemical testing for hypercalcemia, decreased levels of alkaline phosphatase, and radiography to detect findings such as metaphyseal flaring, abnormally large fontanelle, and abnormally wide sutures can be used to diagnose infantile hypophosphatasia. In the less severe forms, or those of later onset, often the only sign is the loss of deciduous teeth.\nPatients with infantile hypophosphatasia, such as the patient described in this case study, may show signs at birth or the signs and symptoms may manifest within the first 6 months of life. Respiratory complications are typical due to a rachitic chest, a finding which may lead to the misdiagnosis of vitamin D deficiency (rickets), especially when coupled with some of the findings on blood chemistry. Hypercalcemia is also typical of patients with hypophosphatasia. Infantile hypophosphatasia usually presents with early failure to thrive, hypotonia, seizures, irritability, anemia, hypercalciuria, and phosphoethanolaminuria. Patients may exhibit blue sclerae, bowed short limbs, metaphyseal cupping, bony spurs on the ulna and fibula, lack of skeletal ossification, easy fracturing, craniosynostosis, and poorly formed teeth.[3]Hypercalcemia and hypercalciuria can cause vomiting and compromise the kidneys. Nephrocalcinosis and seizures may also be present in a patient affected with infantile hypophosphatasia.[4]\nChildhood hypophosphatasia is usually diagnosed by a dentist when premature loss of deciduous teeth is seen. As stated, this may be the only symptom, and other symptoms may be ascribed to other bone diseases. Those affected may also have stunted growth, a waddling gait, and learn to walk later than normal.\nAdult hypophosphatasia presents in 2 forms: a biphasic type, which appears during childhood and is often not diagnosed until it becomes much more active during middle age; and a monophasic form, which appears later in life with milder symptoms. Both of these forms are generally not diagnosed until the fourth decade of life. Tooth loss, metatarsal stress fractures, and femur fractures, which cause hip and thigh pain in adults are indicative of this diagnosis.\nOdontohypophosphatasia presents only as through the loss of deciduous teeth and does not appear with any other symptoms of decreased mineralization.[4]\nOverall, hypophosphatasia is considered quite similar to osteogenesis imperfecta. Many of the symptoms are common to both diseases, such as the blue sclera and the pathologic fractures. Unlike hypophosphatasia, however, osteogenesis imperfecta does not correlate with a decreased alkaline phosphatase level concomitant with an increase in other endogenous substances, such as vitamin B6 and phosphoethanolamine. This patient's endocrinologist also suggested hypervitaminosis D, subcutaneous fat necrosis, Bartter syndrome, Williams syndrome, Jansen syndrome, and embryonal renal tumor as possible causes for the patient's symptoms.\nHypervitaminosis D was ruled out because it was not supported by any of the laboratory findings. Subcutaneous fat necrosis did not account for the radiologic findings. None of the other diagnoses could account for the combination of symptoms seen in this 3-month-old. Hypophosphatasia was indicated clinically, biochemically, and radiologically. The severe failure to thrive, blue sclera, and flaring at the wrists are all typical of the condition. Laboratory analysis showed the expected decreased alkaline phosphatase activity, elevated urinary phosphoethanolamine levels, and high serum levels of vitamin B6. In addition, a fluorescent in situ hybridization (FISH) test for the Williams syndrome deletion was negative. The radiology report that showed metaphyseal flaring and osteopenia further pointed towards a diagnosis of hypophosphatasia.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 3-Month-Old Boy With Severe Failure to Thrive and Hypercalcemia"
},
{
"authors": "Christine A. Ebert-Santos, MD; Anicia Santos",
"content": [
"Several experimental treatments are available for infantile hypophosphatasia, although none have provided a definitive clinical course of action. Bone quality has improved after a bone marrow transplantation in some patients, but it has not reversed the course of the hypophosphatasia. Improvement in technique, such as multiple administration sites, shows promise. Older patients have had some relief using parathyroid hormone therapy, but this may worsen skeletal growth in young patients. Enzyme replacement therapy has been suggested, and when tried it has been shown to help reverse the alkaline phosphatase deficiency, but it does not reverse the hypomineralization of bone and has yet to be of clinical significance.[4] Some patients have benefited from calcitonin injections to control the hypercalcemia, in association with hydrochlorothiazide treatment (a diuretic that increases the absorption of calcium).[1] It appears that some patients who survive infantile hypophosphatasia experience a spontaneous improvement in their clinical condition. It is possible that this is due to the decrease in growth velocity experienced after infancy. Bisphosphonates have been ineffectual and may be contraindicated.",
"The prognosis for the patient discussed here was poor, at best. The condition is considered to be lethal, with death resulting usually secondary to respiratory insufficiency or severe infection. If a patient survives the first few months, it is possible that the condition will spontaneously improve.[5] Initially, the infant in this case was hospitalized to treat the severe failure to thrive, and once his weight had increased slightly, the patient was referred to an endocrinologist. It was at this point that the patient was diagnosed. The family relocated to an area that is relatively lower in elevation above sea level, as compared with their previous residence. The patient was put on a low-calcium formula to control the hypercalcemia, although this cannot reverse the damage already done by the decreased mineralization of his bones. If necessary, hydrocortisone or salmon calcitonin could be added to this treatment. The patient's parents were informed of the possibility of using bone-targeted enzyme replacement therapy to treat the condition as part of a clinical trial, but they have yet to decide whether to exercise this option."
],
"date": "July 17, 2017",
"figures": [],
"markdown": "# A 3-Month-Old Boy With Severe Failure to Thrive and Hypercalcemia\n\n **Authors:** Christine A. Ebert-Santos, MD; Anicia Santos \n **Date:** July 17, 2017\n\n ## Content\n\n Several experimental treatments are available for infantile hypophosphatasia, although none have provided a definitive clinical course of action. Bone quality has improved after a bone marrow transplantation in some patients, but it has not reversed the course of the hypophosphatasia. Improvement in technique, such as multiple administration sites, shows promise. Older patients have had some relief using parathyroid hormone therapy, but this may worsen skeletal growth in young patients. Enzyme replacement therapy has been suggested, and when tried it has been shown to help reverse the alkaline phosphatase deficiency, but it does not reverse the hypomineralization of bone and has yet to be of clinical significance.[4] Some patients have benefited from calcitonin injections to control the hypercalcemia, in association with hydrochlorothiazide treatment (a diuretic that increases the absorption of calcium).[1] It appears that some patients who survive infantile hypophosphatasia experience a spontaneous improvement in their clinical condition. It is possible that this is due to the decrease in growth velocity experienced after infancy. Bisphosphonates have been ineffectual and may be contraindicated.\nThe prognosis for the patient discussed here was poor, at best. The condition is considered to be lethal, with death resulting usually secondary to respiratory insufficiency or severe infection. If a patient survives the first few months, it is possible that the condition will spontaneously improve.[5] Initially, the infant in this case was hospitalized to treat the severe failure to thrive, and once his weight had increased slightly, the patient was referred to an endocrinologist. It was at this point that the patient was diagnosed. The family relocated to an area that is relatively lower in elevation above sea level, as compared with their previous residence. The patient was put on a low-calcium formula to control the hypercalcemia, although this cannot reverse the damage already done by the decreased mineralization of his bones. If necessary, hydrocortisone or salmon calcitonin could be added to this treatment. The patient's parents were informed of the possibility of using bone-targeted enzyme replacement therapy to treat the condition as part of a clinical trial, but they have yet to decide whether to exercise this option.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 343003,
"choiceText": "Abdominal CT scanning",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 343004,
"choiceText": "Ultrasonography",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 343005,
"choiceText": "Maternal serum calcium level",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 343006,
"choiceText": "Maternal alkaline phosphatase level",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Ultrasonography can be used to successfully identify the disorder in a fetus, and the diagnosis can be confirmed with genetic testing. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 96837,
"questionText": "You are caring for a pregnant woman with a history of hypophosphatasia in her family. She would like a test done to assess whether her baby has the disease. Which of the following is most appropriate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 343007,
"choiceText": "High alkaline phosphatase levels",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 343008,
"choiceText": "Hypokalemia and low blood pressure",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 343009,
"choiceText": "Hypercalcemia and decreased alkaline phosphatase",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 343010,
"choiceText": "Increased mineralization",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Hypophosphatasia is the only one of these clinical entities that is associated with low alkaline phosphatase. Williams syndrome is characterized by cardiac, chromosomal, and facial abnormalities. Bartter syndrome typically presents with hypokalemia and low blood pressure. Radiologic findings similar to those seen in rickets (with decreased mineralization) differentiate hypophosphatasia from hypervitaminosis D (which is associated with increased mineralization). Osteogenesis imperfecta presents with high alkaline phosphatase levels. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 96838,
"questionText": "You examine a 3-month-old infant with failure to thrive and suspect that this patient has hypophosphatasia. What laboratory or physiologic abnormalities would support this conclusion?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 3-Month-Old Boy With Severe Failure to Thrive and Hypercalcemia"
},
{
"authors": "Christine A. Ebert-Santos, MD; Anicia Santos",
"content": [],
"date": "July 17, 2017",
"figures": [],
"markdown": "# A 3-Month-Old Boy With Severe Failure to Thrive and Hypercalcemia\n\n **Authors:** Christine A. Ebert-Santos, MD; Anicia Santos \n **Date:** July 17, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 343003,
"choiceText": "Abdominal CT scanning",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 343004,
"choiceText": "Ultrasonography",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 343005,
"choiceText": "Maternal serum calcium level",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 343006,
"choiceText": "Maternal alkaline phosphatase level",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Ultrasonography can be used to successfully identify the disorder in a fetus, and the diagnosis can be confirmed with genetic testing. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 96837,
"questionText": "You are caring for a pregnant woman with a history of hypophosphatasia in her family. She would like a test done to assess whether her baby has the disease. Which of the following is most appropriate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 343007,
"choiceText": "High alkaline phosphatase levels",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 343008,
"choiceText": "Hypokalemia and low blood pressure",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 343009,
"choiceText": "Hypercalcemia and decreased alkaline phosphatase",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 343010,
"choiceText": "Increased mineralization",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Hypophosphatasia is the only one of these clinical entities that is associated with low alkaline phosphatase. Williams syndrome is characterized by cardiac, chromosomal, and facial abnormalities. Bartter syndrome typically presents with hypokalemia and low blood pressure. Radiologic findings similar to those seen in rickets (with decreased mineralization) differentiate hypophosphatasia from hypervitaminosis D (which is associated with increased mineralization). Osteogenesis imperfecta presents with high alkaline phosphatase levels. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 96838,
"questionText": "You examine a 3-month-old infant with failure to thrive and suspect that this patient has hypophosphatasia. What laboratory or physiologic abnormalities would support this conclusion?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 3-Month-Old Boy With Severe Failure to Thrive and Hypercalcemia"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 342999,
"choiceText": "Osteogenesis imperfecta",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 343000,
"choiceText": "Hypophosphatasia",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 343001,
"choiceText": "Bartter syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 343002,
"choiceText": "Vitamin D deficiency (rickets)",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 96836,
"questionText": "Which of the following is the most likely diagnosis? \r\n<br /><br /><em>Hint: Keep in mind the severe hypercalcemia, alkaline phosphatase, and radiology results.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 343003,
"choiceText": "Abdominal CT scanning",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 343004,
"choiceText": "Ultrasonography",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 343005,
"choiceText": "Maternal serum calcium level",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 343006,
"choiceText": "Maternal alkaline phosphatase level",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Ultrasonography can be used to successfully identify the disorder in a fetus, and the diagnosis can be confirmed with genetic testing. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 96837,
"questionText": "You are caring for a pregnant woman with a history of hypophosphatasia in her family. She would like a test done to assess whether her baby has the disease. Which of the following is most appropriate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 343007,
"choiceText": "High alkaline phosphatase levels",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 343008,
"choiceText": "Hypokalemia and low blood pressure",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 343009,
"choiceText": "Hypercalcemia and decreased alkaline phosphatase",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 343010,
"choiceText": "Increased mineralization",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Hypophosphatasia is the only one of these clinical entities that is associated with low alkaline phosphatase. Williams syndrome is characterized by cardiac, chromosomal, and facial abnormalities. Bartter syndrome typically presents with hypokalemia and low blood pressure. Radiologic findings similar to those seen in rickets (with decreased mineralization) differentiate hypophosphatasia from hypervitaminosis D (which is associated with increased mineralization). Osteogenesis imperfecta presents with high alkaline phosphatase levels. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 96838,
"questionText": "You examine a 3-month-old infant with failure to thrive and suspect that this patient has hypophosphatasia. What laboratory or physiologic abnormalities would support this conclusion?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
721158 | /viewarticle/721158 | [
{
"authors": "Somprakas Basu, MS; Vivek Srivastava, MBBS, MS; Mumtaz Ahmad Ansari, MBBS, MS; Anand Kumar, MS",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 26-year-old man presents to the emergency department (ED) complaining of recurrent, colicky abdominal pain, intermittent distention, and vomiting for the past 5 months. His bowel and bladder habits are normal and he denies any hematemesis, melena, hematochezia, or fevers.",
"He reports no history of tuberculosis, significant medical illnesses, or prior abdominal surgeries. His medications include mefenamic acid and dicyclomine injections for pain, which provide symptomatic relief."
],
"date": "July 17, 2017",
"figures": [],
"markdown": "# A 26-Year-Old Man With Abdominal Pain\n\n **Authors:** Somprakas Basu, MS; Vivek Srivastava, MBBS, MS; Mumtaz Ahmad Ansari, MBBS, MS; Anand Kumar, MS \n **Date:** July 17, 2017\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 26-year-old man presents to the emergency department (ED) complaining of recurrent, colicky abdominal pain, intermittent distention, and vomiting for the past 5 months. His bowel and bladder habits are normal and he denies any hematemesis, melena, hematochezia, or fevers.\nHe reports no history of tuberculosis, significant medical illnesses, or prior abdominal surgeries. His medications include mefenamic acid and dicyclomine injections for pain, which provide symptomatic relief.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 26-Year-Old Man With Abdominal Pain"
},
{
"authors": "Somprakas Basu, MS; Vivek Srivastava, MBBS, MS; Mumtaz Ahmad Ansari, MBBS, MS; Anand Kumar, MS",
"content": [
"Upon physical examination, the patient is a well-developed, pale-appearing man noted to be in moderate distress. His vital signs reveal an oral temperature of 98.4°F (36.9°C), pulse of 98 beats/min, blood pressure of 110/70 mm Hg, and respiratory rate of 32 breaths/min. His sclerae are anicteric and his oropharynx is dry.",
"The respiratory and cardiovascular examination findings are normal. The extremities are cool and without any cyanosis or edema. The abdomen is distended but soft and nontender, with visible peristalsis in the central abdomen. Swelling is appreciated in the hypogastrium, which becomes prominent during peristaltic waves. He has hyperactive bowel sounds. No appreciable organomegaly, fluid wave, or palpable hernia is observed. The genitourinary examination findings are normal, and the rectal examination reveals normal tone and soft, yellow stool that is negative for occult blood.",
"The laboratory analysis reveals mild anemia (hemoglobin 9.6 g/dL [96 g/L]), elevated blood urea nitrogen (52 mg/dL [18.6 mmol/L]), and hyponatremia (serum sodium 124 mEq/L [124 mmol/L]). Plain radiography of the abdomen demonstrates multiple air-fluid levels, suggesting a small-bowel obstruction (Figure 1).",
"Figure 1.",
"Abdominal ultrasonography reveals distended small-bowel loops with hyperperistalsis. He is admitted for observation, bowel rest, and intravenous fluid therapy. He is kept NPO, and a nasogastric tube is inserted to decompress the stomach. Intravenous fluid supplementation is started to correct dehydration and sodium loss, and a close watch is kept on his abdominal girth and urinary output. After 10 hours, his symptoms are partially relieved and abdominal distention has decreased; however, he continues to experience colicky abdominal pain (although less frequently). Enteroclysis is performed the next day. It reveals dilated ileal loops and pooling of the contrast in a localized, grossly distended segment of the gut in the lower abdomen (Figure 2).",
"Figure 2."
],
"date": "July 17, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/721/158/721158-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/721/158/721158-thumb2.png"
}
],
"markdown": "# A 26-Year-Old Man With Abdominal Pain\n\n **Authors:** Somprakas Basu, MS; Vivek Srivastava, MBBS, MS; Mumtaz Ahmad Ansari, MBBS, MS; Anand Kumar, MS \n **Date:** July 17, 2017\n\n ## Content\n\n Upon physical examination, the patient is a well-developed, pale-appearing man noted to be in moderate distress. His vital signs reveal an oral temperature of 98.4°F (36.9°C), pulse of 98 beats/min, blood pressure of 110/70 mm Hg, and respiratory rate of 32 breaths/min. His sclerae are anicteric and his oropharynx is dry.\nThe respiratory and cardiovascular examination findings are normal. The extremities are cool and without any cyanosis or edema. The abdomen is distended but soft and nontender, with visible peristalsis in the central abdomen. Swelling is appreciated in the hypogastrium, which becomes prominent during peristaltic waves. He has hyperactive bowel sounds. No appreciable organomegaly, fluid wave, or palpable hernia is observed. The genitourinary examination findings are normal, and the rectal examination reveals normal tone and soft, yellow stool that is negative for occult blood.\nThe laboratory analysis reveals mild anemia (hemoglobin 9.6 g/dL [96 g/L]), elevated blood urea nitrogen (52 mg/dL [18.6 mmol/L]), and hyponatremia (serum sodium 124 mEq/L [124 mmol/L]). Plain radiography of the abdomen demonstrates multiple air-fluid levels, suggesting a small-bowel obstruction (Figure 1).\nFigure 1.\nAbdominal ultrasonography reveals distended small-bowel loops with hyperperistalsis. He is admitted for observation, bowel rest, and intravenous fluid therapy. He is kept NPO, and a nasogastric tube is inserted to decompress the stomach. Intravenous fluid supplementation is started to correct dehydration and sodium loss, and a close watch is kept on his abdominal girth and urinary output. After 10 hours, his symptoms are partially relieved and abdominal distention has decreased; however, he continues to experience colicky abdominal pain (although less frequently). Enteroclysis is performed the next day. It reveals dilated ileal loops and pooling of the contrast in a localized, grossly distended segment of the gut in the lower abdomen (Figure 2).\nFigure 2.\n\n ## Figures\n\n **Figure 1.** \n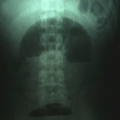 \n\n**Figure 2.** \n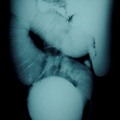 \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 341152,
"choiceText": "Foreign body in the small bowel",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 341153,
"choiceText": "Small-bowel obstruction due to a giant ileal diverticulum",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 341154,
"choiceText": "Inflammatory bowel disease",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 341155,
"choiceText": "Intussusception",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 96162,
"questionText": "What is the diagnosis?<br/><br/>\r\n<em>Hint: Look closely at the distal segment of ileum containing pooled contrast in Figure 2.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 26-Year-Old Man With Abdominal Pain"
},
{
"authors": "Somprakas Basu, MS; Vivek Srivastava, MBBS, MS; Mumtaz Ahmad Ansari, MBBS, MS; Anand Kumar, MS",
"content": [
"In this case, the cause of the small-bowel obstruction was clinically obscure. The presence of distal air (descending colon) on the plain radiograph, as well as the partial relief of symptoms following medical management, indicated an incomplete bowel obstruction. The enteroclysis supported the diagnosis of a giant ileal diverticulum, which was later confirmed at laparotomy.",
"Giant ileal diverticulum is traditionally a disease found in middle-aged and older persons; it remains a rare cause of subacute intestinal obstruction in young individuals. A preoperative, contrast-enhanced CT scan may be useful in making the diagnosis. However, if symptoms of abdominal pain, diarrhea, steatorrhea, or gastrointestinal bleeding persist, a water-soluble contrast follow-through study of the small bowel may still be indicated.[1,2]",
"A diverticulum is an out-pouching from the wall of the gastrointestinal tract and can occur from the stomach to the rectosigmoid colon. Two varieties of diverticula are recognized: In the congenital variety, all layers of the bowel are present on the wall of the diverticulum (eg, Meckel diverticulum); in the acquired variety, the wall on the diverticulum consists of mucosa and submucosa only and lacks a muscular layer. Due to this distinction, the latter is usually referred to as a \"false diverticulum.\"",
"Most small bowel diverticula are thought to be of the latter variety. These diverticula are usually situated on the mesenteric border of the intestine into the mesenteric fat. Acquired jejunoileal diverticulosis was first described by Sommering in 1794 and later in 1807 by Sir Astley Cooper.[3] The true prevalence rate of this rare disease is not known. Autopsy studies report a prevalence rate less than 5% for the jejunoileal variety[4] and 6%-22% for duodenal lesions.[5]",
"Diverticula are usually multiple and tend to be larger and higher in number in the proximal jejunum, whereas distal to the proximal jejunum they tend to be smaller and found in lesser numbers.[6] Simultaneous involvement of both the jejunum and ileum is rare. Males are affected slightly more than females, and the disease is most often seen in adults in the fifth to seventh decades of life.",
"Current hypotheses regarding the etiology of jejunoileal diverticula focus on the abnormalities of smooth muscle and the myenteric plexus. Careful microscopic evaluation of resected specimens indicates 3 types of abnormality in the bowel wall: decreased number of normal muscle cells consistent with progressive systemic sclerosis; visceral myopathy as evidenced by the presence of fibrosis and degenerated smooth muscle cells; and neuronal and axonal degeneration indicative of a visceral neuropathy.[7]",
"Any of these abnormalities alone or in combination could lead to disordered and nonpropulsive smooth muscle contractions, resulting in increased intraluminal pressure and herniation of mucosa and submucosa through the weak mesenteric margin, which is penetrated by blood vessels. These pulsion diverticula (diverticula formed by pressure from within) usually have a narrow mouth with a thin or absent muscle layer. When the muscularis becomes weak or abnormal, the muscle wall of the diverticulum is thinned and fibrosed and the diverticulum are wide-mouthed."
],
"date": "July 17, 2017",
"figures": [],
"markdown": "# A 26-Year-Old Man With Abdominal Pain\n\n **Authors:** Somprakas Basu, MS; Vivek Srivastava, MBBS, MS; Mumtaz Ahmad Ansari, MBBS, MS; Anand Kumar, MS \n **Date:** July 17, 2017\n\n ## Content\n\n In this case, the cause of the small-bowel obstruction was clinically obscure. The presence of distal air (descending colon) on the plain radiograph, as well as the partial relief of symptoms following medical management, indicated an incomplete bowel obstruction. The enteroclysis supported the diagnosis of a giant ileal diverticulum, which was later confirmed at laparotomy.\nGiant ileal diverticulum is traditionally a disease found in middle-aged and older persons; it remains a rare cause of subacute intestinal obstruction in young individuals. A preoperative, contrast-enhanced CT scan may be useful in making the diagnosis. However, if symptoms of abdominal pain, diarrhea, steatorrhea, or gastrointestinal bleeding persist, a water-soluble contrast follow-through study of the small bowel may still be indicated.[1,2]\nA diverticulum is an out-pouching from the wall of the gastrointestinal tract and can occur from the stomach to the rectosigmoid colon. Two varieties of diverticula are recognized: In the congenital variety, all layers of the bowel are present on the wall of the diverticulum (eg, Meckel diverticulum); in the acquired variety, the wall on the diverticulum consists of mucosa and submucosa only and lacks a muscular layer. Due to this distinction, the latter is usually referred to as a \"false diverticulum.\"\nMost small bowel diverticula are thought to be of the latter variety. These diverticula are usually situated on the mesenteric border of the intestine into the mesenteric fat. Acquired jejunoileal diverticulosis was first described by Sommering in 1794 and later in 1807 by Sir Astley Cooper.[3] The true prevalence rate of this rare disease is not known. Autopsy studies report a prevalence rate less than 5% for the jejunoileal variety[4] and 6%-22% for duodenal lesions.[5]\nDiverticula are usually multiple and tend to be larger and higher in number in the proximal jejunum, whereas distal to the proximal jejunum they tend to be smaller and found in lesser numbers.[6] Simultaneous involvement of both the jejunum and ileum is rare. Males are affected slightly more than females, and the disease is most often seen in adults in the fifth to seventh decades of life.\nCurrent hypotheses regarding the etiology of jejunoileal diverticula focus on the abnormalities of smooth muscle and the myenteric plexus. Careful microscopic evaluation of resected specimens indicates 3 types of abnormality in the bowel wall: decreased number of normal muscle cells consistent with progressive systemic sclerosis; visceral myopathy as evidenced by the presence of fibrosis and degenerated smooth muscle cells; and neuronal and axonal degeneration indicative of a visceral neuropathy.[7]\nAny of these abnormalities alone or in combination could lead to disordered and nonpropulsive smooth muscle contractions, resulting in increased intraluminal pressure and herniation of mucosa and submucosa through the weak mesenteric margin, which is penetrated by blood vessels. These pulsion diverticula (diverticula formed by pressure from within) usually have a narrow mouth with a thin or absent muscle layer. When the muscularis becomes weak or abnormal, the muscle wall of the diverticulum is thinned and fibrosed and the diverticulum are wide-mouthed.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 341152,
"choiceText": "Foreign body in the small bowel",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 341153,
"choiceText": "Small-bowel obstruction due to a giant ileal diverticulum",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 341154,
"choiceText": "Inflammatory bowel disease",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 341155,
"choiceText": "Intussusception",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 96162,
"questionText": "What is the diagnosis?<br/><br/>\r\n<em>Hint: Look closely at the distal segment of ileum containing pooled contrast in Figure 2.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 26-Year-Old Man With Abdominal Pain"
},
{
"authors": "Somprakas Basu, MS; Vivek Srivastava, MBBS, MS; Mumtaz Ahmad Ansari, MBBS, MS; Anand Kumar, MS",
"content": [
"Diverticula are silent in most cases (60%-70%) and are incidentally found following a radiologic study or during laparotomy for another disease. Nonspecific chronic abdominal complaints, including crampy pain, postprandial bloating, flatulence and diarrhea, malabsorption, and vitamin B12 deficiency occur as a result of bacterial proliferation inside the diverticulum. Acute complications (8%-30%) are dangerous and often result in urgent laparotomy.[8]",
"Diverticulitis can occur (2%-6% of cases), wherein inflammation is usually the result of a foreign-body inspissation or the presence of an enterolith.[9] If the inflammation is severe, ulceration and hemorrhage may occur and may present as either melena or hematochezia. Small-intestine diverticula should be considered as a cause of severe lower gastrointestinal bleeding in a patient in whom no other source of bleeding is readily identified.",
"In extreme cases, the wall of the diverticulum may undergo ischemic necrosis and become gangrenous. Perforation of a diverticulum results in generalized peritonitis, a localized abscess, or a fistula formation between the adjacent loops of bowel.",
"Small-bowel obstruction can occur as a result of postinflammatory adhesions, bands, or by volvulus of the diverticulum bearing a loop of intestine. Incomplete bowel obstruction may be caused by enteroliths that form in the diverticula, which subsequently become dislodged and obstruct the distal intestine.[9] The clinical picture of intestinal diverticula may be confused with other causes of an acute abdomen (eg, acute appendicitis, cholecystitis, peptic and enteric perforations).",
"Metabolic blind-loop syndrome/small intestine stasis syndrome may occur, along with pernicious anemia (in extreme situations) resulting from vitamin B12 deficiency. Other complications of giant diverticulum include infestation with roundworms or threadworms, neoplastic changes, and pneumatosis cystoids. No single confirmatory diagnostic test for small-bowel diverticulum is recognized. Plain abdominal radiography and/or chest radiography may demonstrate pneumoperitoneum indicating perforation, or multiple air-fluid levels indicating obstruction. A water-soluble contrast follow-through may demonstrate filling of the diverticulum.",
"In the case of mesenteric abscess formation, small bowel loops are displaced by the mass. On enteroclysis (the most sensitive contrast examination of the small bowel), contrast may be seen to pass back and forth from the intestinal lumen into the diverticulum. This condition may be associated with hypertrophy and dilatation of the portion of bowel proximal to the diverticulum. Findings on abdominal CT scans include jejunoileal wall thickening, mesenteric thickening and inflammation, free gas, and fluid collection. A phlegmon can be identified in the mesentery of the retroperitoneal space, providing an initial clue to a possible small-intestine diverticular disease. Direct visualization of the diverticulum is also possible using CT.[10]",
"An esophagogastroduodenoscopic examination is not useful in acute diverticulitis of the small bowel, as jejunal or ileal diverticula are not accessible by this approach. A colonoscopic examination is useful in evaluating the source of bleeding and excluding other diagnoses; however, small-bowel diverticula are generally not accessible to colonoscopy. These diverticula can be investigated by enteroscopic examination using capsule enteroscopy and double-balloon enteroscopy.[11] The limitation of capsule enteroscopy lies in its inability to localize with accuracy the region of the small bowel involved. In double-balloon enteroscopy, an experienced operator is required to accurately recognize these lesions.",
"Both investigations are of limited value in emergent situations, such as is depicted in this case. As a result, the diagnosis depends on exclusion; therefore, it is seldom made before laparotomy. In nonacute but symptomatic conditions, diagnostic laparoscopy plays an important role in ensuring an accurate diagnosis in most cases and avoiding unnecessary laparotomy.[7]"
],
"date": "July 17, 2017",
"figures": [],
"markdown": "# A 26-Year-Old Man With Abdominal Pain\n\n **Authors:** Somprakas Basu, MS; Vivek Srivastava, MBBS, MS; Mumtaz Ahmad Ansari, MBBS, MS; Anand Kumar, MS \n **Date:** July 17, 2017\n\n ## Content\n\n Diverticula are silent in most cases (60%-70%) and are incidentally found following a radiologic study or during laparotomy for another disease. Nonspecific chronic abdominal complaints, including crampy pain, postprandial bloating, flatulence and diarrhea, malabsorption, and vitamin B12 deficiency occur as a result of bacterial proliferation inside the diverticulum. Acute complications (8%-30%) are dangerous and often result in urgent laparotomy.[8]\nDiverticulitis can occur (2%-6% of cases), wherein inflammation is usually the result of a foreign-body inspissation or the presence of an enterolith.[9] If the inflammation is severe, ulceration and hemorrhage may occur and may present as either melena or hematochezia. Small-intestine diverticula should be considered as a cause of severe lower gastrointestinal bleeding in a patient in whom no other source of bleeding is readily identified.\nIn extreme cases, the wall of the diverticulum may undergo ischemic necrosis and become gangrenous. Perforation of a diverticulum results in generalized peritonitis, a localized abscess, or a fistula formation between the adjacent loops of bowel.\nSmall-bowel obstruction can occur as a result of postinflammatory adhesions, bands, or by volvulus of the diverticulum bearing a loop of intestine. Incomplete bowel obstruction may be caused by enteroliths that form in the diverticula, which subsequently become dislodged and obstruct the distal intestine.[9] The clinical picture of intestinal diverticula may be confused with other causes of an acute abdomen (eg, acute appendicitis, cholecystitis, peptic and enteric perforations).\nMetabolic blind-loop syndrome/small intestine stasis syndrome may occur, along with pernicious anemia (in extreme situations) resulting from vitamin B12 deficiency. Other complications of giant diverticulum include infestation with roundworms or threadworms, neoplastic changes, and pneumatosis cystoids. No single confirmatory diagnostic test for small-bowel diverticulum is recognized. Plain abdominal radiography and/or chest radiography may demonstrate pneumoperitoneum indicating perforation, or multiple air-fluid levels indicating obstruction. A water-soluble contrast follow-through may demonstrate filling of the diverticulum.\nIn the case of mesenteric abscess formation, small bowel loops are displaced by the mass. On enteroclysis (the most sensitive contrast examination of the small bowel), contrast may be seen to pass back and forth from the intestinal lumen into the diverticulum. This condition may be associated with hypertrophy and dilatation of the portion of bowel proximal to the diverticulum. Findings on abdominal CT scans include jejunoileal wall thickening, mesenteric thickening and inflammation, free gas, and fluid collection. A phlegmon can be identified in the mesentery of the retroperitoneal space, providing an initial clue to a possible small-intestine diverticular disease. Direct visualization of the diverticulum is also possible using CT.[10]\nAn esophagogastroduodenoscopic examination is not useful in acute diverticulitis of the small bowel, as jejunal or ileal diverticula are not accessible by this approach. A colonoscopic examination is useful in evaluating the source of bleeding and excluding other diagnoses; however, small-bowel diverticula are generally not accessible to colonoscopy. These diverticula can be investigated by enteroscopic examination using capsule enteroscopy and double-balloon enteroscopy.[11] The limitation of capsule enteroscopy lies in its inability to localize with accuracy the region of the small bowel involved. In double-balloon enteroscopy, an experienced operator is required to accurately recognize these lesions.\nBoth investigations are of limited value in emergent situations, such as is depicted in this case. As a result, the diagnosis depends on exclusion; therefore, it is seldom made before laparotomy. In nonacute but symptomatic conditions, diagnostic laparoscopy plays an important role in ensuring an accurate diagnosis in most cases and avoiding unnecessary laparotomy.[7]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 26-Year-Old Man With Abdominal Pain"
},
{
"authors": "Somprakas Basu, MS; Vivek Srivastava, MBBS, MS; Mumtaz Ahmad Ansari, MBBS, MS; Anand Kumar, MS",
"content": [
"The mere presence of a diverticulum does not justify its surgical removal. If the diverticula are large and found in an isolated, dilated, and hypertrophied segment, and if they are the cause of intestinal obstruction, hemorrhage, adhesions, perforation, or intra-abdominal or retroperitoneal abscess formation, then they should be removed.",
"Patients with steatorrhea or pernicious anemia should be treated initially with a course of antibiotics as well as vitamin B12 and folate supplementation plus correction of the anemia. If a patient fails to respond to medical therapy, laparoscopy or laparotomy for resection should be considered. A single diverticulum is best treated by diverticulectomy. Inversions of small lesions are best avoided because these can create a lead point for intussusception. When the diverticula are confined to a segment of the intestine, resection with restoration of bowel continuity should be the goal.",
"Multiple diverticula scattered throughout the small bowel present a difficult treatment scenario. In order to avoid short bowel syndrome or malabsorption, resection should be restricted to those segments containing the largest diverticula or those producing complications such as perforation, abscess, and bowel adhesions.",
"In the case above, the patient underwent an exploratory laparotomy which revealed a single, giant, wide-mouthed diverticulum of 15 cm in diameter in the distal ileum with patent proximal and distal lumen (Figure 3).",
"Figure 3.",
"The ileal and distal jejunal loops were distended and aperistaltic. The diverticulum was thick-walled and inflamed, and it adhered to an adjacent loop of bowel. It did not show any evidence of perforation or gangrene. A segmental ileal resection including the diverticulum was performed, with end-to-end primary anastomosis. A thorough search of the peritoneal cavity did not reveal any other abnormalities. The postoperative course was uneventful. The histopathological examination of the resected specimen demonstrated a diverticulum with small-intestinal mucosa with mucous glands metaplasia, areas of ulceration, and acute inflammatory cell infiltration to the bowel wall, without recognizable nerve plexus. All of the above are suggestive of a heavily inflamed diverticulum with early infarction. After 1 year follow-up, the patient remains asymptomatic."
],
"date": "July 17, 2017",
"figures": [
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/721/158/721158-thumb3.png"
}
],
"markdown": "# A 26-Year-Old Man With Abdominal Pain\n\n **Authors:** Somprakas Basu, MS; Vivek Srivastava, MBBS, MS; Mumtaz Ahmad Ansari, MBBS, MS; Anand Kumar, MS \n **Date:** July 17, 2017\n\n ## Content\n\n The mere presence of a diverticulum does not justify its surgical removal. If the diverticula are large and found in an isolated, dilated, and hypertrophied segment, and if they are the cause of intestinal obstruction, hemorrhage, adhesions, perforation, or intra-abdominal or retroperitoneal abscess formation, then they should be removed.\nPatients with steatorrhea or pernicious anemia should be treated initially with a course of antibiotics as well as vitamin B12 and folate supplementation plus correction of the anemia. If a patient fails to respond to medical therapy, laparoscopy or laparotomy for resection should be considered. A single diverticulum is best treated by diverticulectomy. Inversions of small lesions are best avoided because these can create a lead point for intussusception. When the diverticula are confined to a segment of the intestine, resection with restoration of bowel continuity should be the goal.\nMultiple diverticula scattered throughout the small bowel present a difficult treatment scenario. In order to avoid short bowel syndrome or malabsorption, resection should be restricted to those segments containing the largest diverticula or those producing complications such as perforation, abscess, and bowel adhesions.\nIn the case above, the patient underwent an exploratory laparotomy which revealed a single, giant, wide-mouthed diverticulum of 15 cm in diameter in the distal ileum with patent proximal and distal lumen (Figure 3).\nFigure 3.\nThe ileal and distal jejunal loops were distended and aperistaltic. The diverticulum was thick-walled and inflamed, and it adhered to an adjacent loop of bowel. It did not show any evidence of perforation or gangrene. A segmental ileal resection including the diverticulum was performed, with end-to-end primary anastomosis. A thorough search of the peritoneal cavity did not reveal any other abnormalities. The postoperative course was uneventful. The histopathological examination of the resected specimen demonstrated a diverticulum with small-intestinal mucosa with mucous glands metaplasia, areas of ulceration, and acute inflammatory cell infiltration to the bowel wall, without recognizable nerve plexus. All of the above are suggestive of a heavily inflamed diverticulum with early infarction. After 1 year follow-up, the patient remains asymptomatic.\n\n ## Figures\n\n **Figure 3.** \n \n\n\n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 341160,
"choiceText": "Small bowel diverticula are usually asymptomatic",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 341161,
"choiceText": "Small bowel diverticula are commonly situated in the antimesenteric border of the intestine",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 341162,
"choiceText": "Giant ileal diverticulum is a disease typically found in middle-aged to elderly patients",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 341163,
"choiceText": "Malabsorption syndrome is a common complication",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The small bowel diverticula are commonly protruding towards the mesenteric border of the gut. The presence of myopathy or neuropathy of the muscular coat of the small bowel leads to discoordinated, nonpropulsive bowel movements and increased intraluminal pressure. This causes herniation of the mucosal and submucosal layers through the mesenteric margin, which is a relatively weak zone due to penetration of mesenteric blood vessels. The other statements are true.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 96164,
"questionText": "You are caring for a 45-year-old man with nonspecific, subacute abdominal symptoms. You consider the diagnosis of small bowel diverticulum as a possible cause of his symptoms. Which of the following statements about small bowel diverticulum is not true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 341168,
"choiceText": "Surgical resection is recommended for all small bowel diverticula, and the patient should receive urgent surgical consultation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 341169,
"choiceText": "If the patient has evidence of steatorrhea or pernicious anemia, he should initially receive a course of antibiotics and vitamins",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 341170,
"choiceText": "Intestinal obstruction, abscess formation, and hemorrhage are contraindications for surgical resection of small bowel diverticula",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 341171,
"choiceText": "Colonoscopy is the preferred method of removing small bowel diverticula",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The mere presence of a diverticulum does not justify its surgical removal. If a single diverticulum is large and isolated, dilated, and found in a hypertrophied segment or if it is the cause of intestinal obstruction, hemorrhage, adhesions, perforation, or intra-abdominal or retroperitoneal abscess formation, then it should be removed. Patients with steatorrhea or pernicious anemia should be treated initially with a course of antibiotics as well as vitamin B12 and folate supplementation plus correction of the anemia before an operation. In patients who fail to respond to medical therapy, laparoscopic or open removal of the diverticula should be considered. Small bowel diverticula are not amenable to upper or lower endoscopic treatment.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 96166,
"questionText": "You obtain abdominal imaging for this patient, which supports the diagnosis of a small bowel diverticulum. You are now considering treatment options for your patient. Which of the following statements is true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 26-Year-Old Man With Abdominal Pain"
},
{
"authors": "Somprakas Basu, MS; Vivek Srivastava, MBBS, MS; Mumtaz Ahmad Ansari, MBBS, MS; Anand Kumar, MS",
"content": [],
"date": "July 17, 2017",
"figures": [],
"markdown": "# A 26-Year-Old Man With Abdominal Pain\n\n **Authors:** Somprakas Basu, MS; Vivek Srivastava, MBBS, MS; Mumtaz Ahmad Ansari, MBBS, MS; Anand Kumar, MS \n **Date:** July 17, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 341160,
"choiceText": "Small bowel diverticula are usually asymptomatic",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 341161,
"choiceText": "Small bowel diverticula are commonly situated in the antimesenteric border of the intestine",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 341162,
"choiceText": "Giant ileal diverticulum is a disease typically found in middle-aged to elderly patients",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 341163,
"choiceText": "Malabsorption syndrome is a common complication",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The small bowel diverticula are commonly protruding towards the mesenteric border of the gut. The presence of myopathy or neuropathy of the muscular coat of the small bowel leads to discoordinated, nonpropulsive bowel movements and increased intraluminal pressure. This causes herniation of the mucosal and submucosal layers through the mesenteric margin, which is a relatively weak zone due to penetration of mesenteric blood vessels. The other statements are true.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 96164,
"questionText": "You are caring for a 45-year-old man with nonspecific, subacute abdominal symptoms. You consider the diagnosis of small bowel diverticulum as a possible cause of his symptoms. Which of the following statements about small bowel diverticulum is not true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 341168,
"choiceText": "Surgical resection is recommended for all small bowel diverticula, and the patient should receive urgent surgical consultation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 341169,
"choiceText": "If the patient has evidence of steatorrhea or pernicious anemia, he should initially receive a course of antibiotics and vitamins",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 341170,
"choiceText": "Intestinal obstruction, abscess formation, and hemorrhage are contraindications for surgical resection of small bowel diverticula",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 341171,
"choiceText": "Colonoscopy is the preferred method of removing small bowel diverticula",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The mere presence of a diverticulum does not justify its surgical removal. If a single diverticulum is large and isolated, dilated, and found in a hypertrophied segment or if it is the cause of intestinal obstruction, hemorrhage, adhesions, perforation, or intra-abdominal or retroperitoneal abscess formation, then it should be removed. Patients with steatorrhea or pernicious anemia should be treated initially with a course of antibiotics as well as vitamin B12 and folate supplementation plus correction of the anemia before an operation. In patients who fail to respond to medical therapy, laparoscopic or open removal of the diverticula should be considered. Small bowel diverticula are not amenable to upper or lower endoscopic treatment.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 96166,
"questionText": "You obtain abdominal imaging for this patient, which supports the diagnosis of a small bowel diverticulum. You are now considering treatment options for your patient. Which of the following statements is true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 26-Year-Old Man With Abdominal Pain"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 341152,
"choiceText": "Foreign body in the small bowel",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 341153,
"choiceText": "Small-bowel obstruction due to a giant ileal diverticulum",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 341154,
"choiceText": "Inflammatory bowel disease",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 341155,
"choiceText": "Intussusception",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 96162,
"questionText": "What is the diagnosis?<br/><br/>\r\n<em>Hint: Look closely at the distal segment of ileum containing pooled contrast in Figure 2.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 341160,
"choiceText": "Small bowel diverticula are usually asymptomatic",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 341161,
"choiceText": "Small bowel diverticula are commonly situated in the antimesenteric border of the intestine",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 341162,
"choiceText": "Giant ileal diverticulum is a disease typically found in middle-aged to elderly patients",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 341163,
"choiceText": "Malabsorption syndrome is a common complication",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The small bowel diverticula are commonly protruding towards the mesenteric border of the gut. The presence of myopathy or neuropathy of the muscular coat of the small bowel leads to discoordinated, nonpropulsive bowel movements and increased intraluminal pressure. This causes herniation of the mucosal and submucosal layers through the mesenteric margin, which is a relatively weak zone due to penetration of mesenteric blood vessels. The other statements are true.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 96164,
"questionText": "You are caring for a 45-year-old man with nonspecific, subacute abdominal symptoms. You consider the diagnosis of small bowel diverticulum as a possible cause of his symptoms. Which of the following statements about small bowel diverticulum is not true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 341168,
"choiceText": "Surgical resection is recommended for all small bowel diverticula, and the patient should receive urgent surgical consultation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 341169,
"choiceText": "If the patient has evidence of steatorrhea or pernicious anemia, he should initially receive a course of antibiotics and vitamins",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 341170,
"choiceText": "Intestinal obstruction, abscess formation, and hemorrhage are contraindications for surgical resection of small bowel diverticula",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 341171,
"choiceText": "Colonoscopy is the preferred method of removing small bowel diverticula",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The mere presence of a diverticulum does not justify its surgical removal. If a single diverticulum is large and isolated, dilated, and found in a hypertrophied segment or if it is the cause of intestinal obstruction, hemorrhage, adhesions, perforation, or intra-abdominal or retroperitoneal abscess formation, then it should be removed. Patients with steatorrhea or pernicious anemia should be treated initially with a course of antibiotics as well as vitamin B12 and folate supplementation plus correction of the anemia before an operation. In patients who fail to respond to medical therapy, laparoscopic or open removal of the diverticula should be considered. Small bowel diverticula are not amenable to upper or lower endoscopic treatment.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 96166,
"questionText": "You obtain abdominal imaging for this patient, which supports the diagnosis of a small bowel diverticulum. You are now considering treatment options for your patient. Which of the following statements is true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
720939 | /viewarticle/720939 | [
{
"authors": "Jaime Shalkow, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"Background",
"A 17-day-old African boy is brought to an emergency department (ED) in his local area in respiratory distress. For the past 7 days, he has had a nonproductive cough, tactile fevers, rapid breathing, and poor feeding. His parents also note that on 2 occasions when he was short of breath, his lips and fingers turned blue. These episodes lasted less than 30 seconds, were self-resolving, and were not associated with any loss of consciousness. He has not previously seen a doctor. The parents state that they have brought the child to the ED because he seems to be worsening; the second episode in which the patient turned blue occurred earlier today. He is not taking any medications and does not have any known drug allergies. Both parents work as farmers in East Africa. He has 4 older siblings, all without any known medical conditions. The patient was born full-term and was delivered vaginally at home, without any complications. His birth size of 19.3 in (49 cm) and weight of 6.4 lb (2.9 kg) are normal. Of note, the mother received minimal prenatal care."
],
"date": "June 23, 2016",
"figures": [],
"markdown": "# Dyspnea With Intermittent Cyanosis in a Neonate\n\n **Authors:** Jaime Shalkow, MD \n **Date:** June 23, 2016\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nBackground\nA 17-day-old African boy is brought to an emergency department (ED) in his local area in respiratory distress. For the past 7 days, he has had a nonproductive cough, tactile fevers, rapid breathing, and poor feeding. His parents also note that on 2 occasions when he was short of breath, his lips and fingers turned blue. These episodes lasted less than 30 seconds, were self-resolving, and were not associated with any loss of consciousness. He has not previously seen a doctor. The parents state that they have brought the child to the ED because he seems to be worsening; the second episode in which the patient turned blue occurred earlier today. He is not taking any medications and does not have any known drug allergies. Both parents work as farmers in East Africa. He has 4 older siblings, all without any known medical conditions. The patient was born full-term and was delivered vaginally at home, without any complications. His birth size of 19.3 in (49 cm) and weight of 6.4 lb (2.9 kg) are normal. Of note, the mother received minimal prenatal care.\n\n ## Figures\n\n \n*Page 1 of 5*",
"pagination": {
"current_page": 1,
"total_pages": 5
},
"questionnaire": [],
"title": "Dyspnea With Intermittent Cyanosis in a Neonate"
},
{
"authors": "Jaime Shalkow, MD",
"content": [
"Physical Examination and Work-up",
"On physical examination, the patient is awake, alert, reactive, and appears to be well nourished. He is, however, notably pale and in obvious respiratory distress. He has a temperature of 99.5°F (37.5°C), a heart rate of 160 beats/min, a respiratory rate of 56 breaths/min, a blood pressure of 87/55 mm Hg, and an oxygen saturation of 87% while breathing room air. The anterior fontanelle is normal to palpation, and no congenital abnormalities are noted on inspection of the face and head. The neck is short, the trachea is in the central position, and no jugular vein distention is appreciated. No masses, enlarged lymph nodes, or congenital defects are seen.",
"The patient's respiratory movements are rapid, with intercostal and subxiphoidal retractions. The heart sounds are strong, with a regular rate and rhythm and no audible murmur. The left hemithorax is clear to auscultation, without rales or wheezes; however, decreased air entry, dullness to percussion, and diffuse rhonchi are noted in the right side. The abdomen is scaphoid, soft, and nontender, with positive bowel sounds. No masses are palpated. The external genitalia appear normal for the patient's age and sex. The extremities show good range of motion, with a capillary refill of 3 seconds. No other abnormalities are detected on the physical examination.",
"Figure 1.",
"Figure 2.",
"A complete blood cell count reveals 13.0 × 103/µL leukocytes (13.0 × 109/L, with 45% neutrophils [0.45], 42% lymphocytes [0.42], 3% eosinophils [0.03], and no bands). No blood gas analysis is available. Chest radiography is performed (Figure 1) as is a follow-up upper gastrointestinal contrast study (Figure 2). During the fluoroscopic evaluation, the right hemidiaphragm appears motionless.",
"While still in the ED, the patient is intubated for his respiratory distress and intravenous fluids are started. He is then admitted to the neonatal intensive care unit, where he is placed in an incubator. An orogastric tube and a urine catheter are passed, and broad-coverage antibiotics are started."
],
"date": "June 23, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/720/939/720939-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/720/939/720939-thumb2.png"
}
],
"markdown": "# Dyspnea With Intermittent Cyanosis in a Neonate\n\n **Authors:** Jaime Shalkow, MD \n **Date:** June 23, 2016\n\n ## Content\n\n Physical Examination and Work-up\nOn physical examination, the patient is awake, alert, reactive, and appears to be well nourished. He is, however, notably pale and in obvious respiratory distress. He has a temperature of 99.5°F (37.5°C), a heart rate of 160 beats/min, a respiratory rate of 56 breaths/min, a blood pressure of 87/55 mm Hg, and an oxygen saturation of 87% while breathing room air. The anterior fontanelle is normal to palpation, and no congenital abnormalities are noted on inspection of the face and head. The neck is short, the trachea is in the central position, and no jugular vein distention is appreciated. No masses, enlarged lymph nodes, or congenital defects are seen.\nThe patient's respiratory movements are rapid, with intercostal and subxiphoidal retractions. The heart sounds are strong, with a regular rate and rhythm and no audible murmur. The left hemithorax is clear to auscultation, without rales or wheezes; however, decreased air entry, dullness to percussion, and diffuse rhonchi are noted in the right side. The abdomen is scaphoid, soft, and nontender, with positive bowel sounds. No masses are palpated. The external genitalia appear normal for the patient's age and sex. The extremities show good range of motion, with a capillary refill of 3 seconds. No other abnormalities are detected on the physical examination.\nFigure 1.\nFigure 2.\nA complete blood cell count reveals 13.0 × 103/µL leukocytes (13.0 × 109/L, with 45% neutrophils [0.45], 42% lymphocytes [0.42], 3% eosinophils [0.03], and no bands). No blood gas analysis is available. Chest radiography is performed (Figure 1) as is a follow-up upper gastrointestinal contrast study (Figure 2). During the fluoroscopic evaluation, the right hemidiaphragm appears motionless.\nWhile still in the ED, the patient is intubated for his respiratory distress and intravenous fluids are started. He is then admitted to the neonatal intensive care unit, where he is placed in an incubator. An orogastric tube and a urine catheter are passed, and broad-coverage antibiotics are started.\n\n ## Figures\n\n **Figure 1.** \n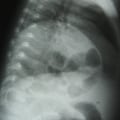 \n\n**Figure 2.** \n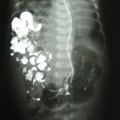 \n\n\n*Page 2 of 5*",
"pagination": {
"current_page": 2,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 340091,
"choiceText": "Pericardial cyst",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 340092,
"choiceText": "Bochdalek hernia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 340093,
"choiceText": "Traumatic diaphragmatic rupture",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 340094,
"choiceText": "Diaphragmatic eventration",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 95820,
"questionText": "What is the reason for this neonate's respiratory distress?<br/>\r\n<br/>\r\n<em>Hint: Pay attention to the chest examination and look closely at the right diaphragm and hemithorax.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Dyspnea With Intermittent Cyanosis in a Neonate"
},
{
"authors": "Jaime Shalkow, MD",
"content": [
"Diaphragmatic eventration results from underdevelopment and thinning of the central portion of one of the hemidiaphragms,[1] which causes a chronically elevated resting position. Diaphragmatic eventration may be congenital or acquired secondary to a phrenic nerve injury.[2] Unlike a diaphragmatic hernia, in which a physical breach of the diaphragm occurs and abdominal contents may herniate into the thorax, the thorax and the peritoneum remain separated in diaphragmatic eventration.[1] Aplasia of the diaphragmatic muscle in diaphragmatic eventration may result in an elevated position of all or part of the diaphragmatic muscle. The diagnosis should be suspected when bowel sounds are auscultated in the thorax of a newborn; it is confirmed with radiologic studies.[3]",
"The classic clinical findings of diaphragmatic eventration include respiratory distress, cyanosis, tachypnea, and diminished breath sounds. A scaphoid abdomen should also direct the clinician's attention toward this entity because abdominal contents rise into the chest. Congenital diaphragmatic eventration may be apparent at birth, but delayed presentations may also occur. Patients may present with wheezing, recurrent respiratory infections (pneumonia, bronchitis, or bronchiectasis), and/or exercise intolerance (dyspnea during feeding); however, even large eventrations might be completely asymptomatic. Prominence of the ipsilateral chest and cardiac apical beat under the sternum or displaced to the opposite side (mediastinal shift) are also frequent findings.[2] Gastric volvulus with significant morbidity and mortality have been described in patients with diaphragmatic anomalies.[4]",
"Chest radiography is the initial study of choice. Posteroanterior and lateral films will show an abnormally elevated diaphragm. In the absence of disease, the line formed by the diaphragm on chest radiography is continuous. When the diaphragm cannot be fully visualized, a pathologic condition may still exist in the appropriate clinical presentation. The affected side of the diaphragm should be at least 2 intercostal spaces higher than the normal side. Another typical finding is the presence of bowel loops in the region of what should be the thorax. A blood gas analysis may reveal respiratory acidosis. The usual confirmatory study is an upper gastrointestinal series, which will demonstrate the presence of bowel loops in what appears to be the chest. CT scanning and MRI can also demonstrate this finding. The criterion standard for making the diagnosis of diaphragmatic eventration, however, is confirmation of a motionless or paradoxically moving hemidiaphragm,[3] which can be done with fluoroscopy or with real-time ultrasonography. Paradoxical diaphragmatic movements can be seen on ultrasound as early as the 17 weeks' gestation, with high specificity for congenital diaphragmatic hernias and eventrations.[5] Additionally, ultrasonography does not expose the patient to ionizing radiation.",
"Diaphragmatic eventration may be congenital or acquired. Congenital diaphragmatic eventration is less common than the acquired variety, and it refers to a thinned central portion of the diaphragm on the affected side. It appears to be secondary to the lack of proper development of or cell migration from the 4 elements forming this structure, which are the septum transversum, the pleuroperitoneal folds, the body wall, and the dorsal root of the mesentery. The exact etiology of the abnormality is unknown, although it may be associated with fetal rubella or cytomegalovirus infection. No familial predisposition is noted. The acquired form, which may be clinically indistinguishable from the congenital form, is usually the result of trauma. When seen in newborns, it is secondary to birth trauma (generally from damage to the phrenic nerve); this may be caused by excessive traction to the arm during delivery. The diaphragm eventually becomes atrophic secondary to the phrenic nerve injury and disuse, and it may develop an eventration. The phrenic nerve is formed by the roots of the cervical motor neurons C3, C4, and C5 in the neck. The acquired form can also be seen in older patients after surgical trauma to the phrenic nerve. It may also be associated with Werdnig-Hoffman disease (spinal muscle atrophy), in which the progressive and generalized loss of neurologic function leads to eventration.",
"On surgical exploration, congenital and acquired forms can be distinguished, because whereas a congenital diaphragmatic eventration has a greatly thinned central portion of the affected diaphragm, an acquired diaphragmatic eventration (if operated on early) has a normal-appearing, but weakened, muscle.[2]"
],
"date": "June 23, 2016",
"figures": [],
"markdown": "# Dyspnea With Intermittent Cyanosis in a Neonate\n\n **Authors:** Jaime Shalkow, MD \n **Date:** June 23, 2016\n\n ## Content\n\n Diaphragmatic eventration results from underdevelopment and thinning of the central portion of one of the hemidiaphragms,[1] which causes a chronically elevated resting position. Diaphragmatic eventration may be congenital or acquired secondary to a phrenic nerve injury.[2] Unlike a diaphragmatic hernia, in which a physical breach of the diaphragm occurs and abdominal contents may herniate into the thorax, the thorax and the peritoneum remain separated in diaphragmatic eventration.[1] Aplasia of the diaphragmatic muscle in diaphragmatic eventration may result in an elevated position of all or part of the diaphragmatic muscle. The diagnosis should be suspected when bowel sounds are auscultated in the thorax of a newborn; it is confirmed with radiologic studies.[3]\nThe classic clinical findings of diaphragmatic eventration include respiratory distress, cyanosis, tachypnea, and diminished breath sounds. A scaphoid abdomen should also direct the clinician's attention toward this entity because abdominal contents rise into the chest. Congenital diaphragmatic eventration may be apparent at birth, but delayed presentations may also occur. Patients may present with wheezing, recurrent respiratory infections (pneumonia, bronchitis, or bronchiectasis), and/or exercise intolerance (dyspnea during feeding); however, even large eventrations might be completely asymptomatic. Prominence of the ipsilateral chest and cardiac apical beat under the sternum or displaced to the opposite side (mediastinal shift) are also frequent findings.[2] Gastric volvulus with significant morbidity and mortality have been described in patients with diaphragmatic anomalies.[4]\nChest radiography is the initial study of choice. Posteroanterior and lateral films will show an abnormally elevated diaphragm. In the absence of disease, the line formed by the diaphragm on chest radiography is continuous. When the diaphragm cannot be fully visualized, a pathologic condition may still exist in the appropriate clinical presentation. The affected side of the diaphragm should be at least 2 intercostal spaces higher than the normal side. Another typical finding is the presence of bowel loops in the region of what should be the thorax. A blood gas analysis may reveal respiratory acidosis. The usual confirmatory study is an upper gastrointestinal series, which will demonstrate the presence of bowel loops in what appears to be the chest. CT scanning and MRI can also demonstrate this finding. The criterion standard for making the diagnosis of diaphragmatic eventration, however, is confirmation of a motionless or paradoxically moving hemidiaphragm,[3] which can be done with fluoroscopy or with real-time ultrasonography. Paradoxical diaphragmatic movements can be seen on ultrasound as early as the 17 weeks' gestation, with high specificity for congenital diaphragmatic hernias and eventrations.[5] Additionally, ultrasonography does not expose the patient to ionizing radiation.\nDiaphragmatic eventration may be congenital or acquired. Congenital diaphragmatic eventration is less common than the acquired variety, and it refers to a thinned central portion of the diaphragm on the affected side. It appears to be secondary to the lack of proper development of or cell migration from the 4 elements forming this structure, which are the septum transversum, the pleuroperitoneal folds, the body wall, and the dorsal root of the mesentery. The exact etiology of the abnormality is unknown, although it may be associated with fetal rubella or cytomegalovirus infection. No familial predisposition is noted. The acquired form, which may be clinically indistinguishable from the congenital form, is usually the result of trauma. When seen in newborns, it is secondary to birth trauma (generally from damage to the phrenic nerve); this may be caused by excessive traction to the arm during delivery. The diaphragm eventually becomes atrophic secondary to the phrenic nerve injury and disuse, and it may develop an eventration. The phrenic nerve is formed by the roots of the cervical motor neurons C3, C4, and C5 in the neck. The acquired form can also be seen in older patients after surgical trauma to the phrenic nerve. It may also be associated with Werdnig-Hoffman disease (spinal muscle atrophy), in which the progressive and generalized loss of neurologic function leads to eventration.\nOn surgical exploration, congenital and acquired forms can be distinguished, because whereas a congenital diaphragmatic eventration has a greatly thinned central portion of the affected diaphragm, an acquired diaphragmatic eventration (if operated on early) has a normal-appearing, but weakened, muscle.[2]\n\n ## Figures\n\n \n*Page 3 of 5*",
"pagination": {
"current_page": 3,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 340091,
"choiceText": "Pericardial cyst",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 340092,
"choiceText": "Bochdalek hernia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 340093,
"choiceText": "Traumatic diaphragmatic rupture",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 340094,
"choiceText": "Diaphragmatic eventration",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 95820,
"questionText": "What is the reason for this neonate's respiratory distress?<br/>\r\n<br/>\r\n<em>Hint: Pay attention to the chest examination and look closely at the right diaphragm and hemithorax.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Dyspnea With Intermittent Cyanosis in a Neonate"
},
{
"authors": "Jaime Shalkow, MD",
"content": [
"Treatment of diaphragmatic eventrations will vary, mostly according to the size of the eventration and the clinical status of the patient. Small eventrations can be left untreated, but large ones require repair, even when asymptomatic, in order to allow for proper lung development. Untreated diaphragmatic dysfunction causes a restrictive deficit with reduced lung volumes, smaller tidal volumes, and increased work of breathing. Patients with respiratory distress need prompt attention and supportive care with endotracheal intubation and ventilation.",
"Patients should not have oral intake. A nasogastric tube should be passed to decompress the stomach, and intravenous fluids should be initiated. Once the patient is stable, surgery is indicated. Repair can be done through the abdomen or the chest; depending on the experience of the surgeon, the approach may be open or minimally invasive.[6] Robotic repair has been shown to be safe and effective.[7] A thoracotomy or thoracoscopy is usually preferred, except when concomitant abdominal abnormalities, such as malrotation, are suspected.",
"Multiple nonabsorbable sutures are used in a staggered fashion across the diaphragm to pull and flatten the muscle. This plication maximizes the intrathoracic space by imbricating the diaphragm to a near horizontal position. Generally, chest tubes are maintained for up to several days and rapid clinical improvement is observed; however, other techniques, such as evacuation of air while closing the intercostal space, have also been described.",
"The differential diagnosis of diaphragmatic eventration includes congenital diaphragmatic hernias (also known as Bochdalek hernias). These hernias occur most commonly on the left side (90%), and the diaphragm itself is not normally visualized in radiographic examinations (except when a thick hernial sac is present).[1,2] Diaphragmatic eventrations can occur on either side of the chest, and patients are usually asymptomatic for longer periods of time because they lack the pulmonary hypoplasia associated with diaphragmatic hernias.[2] When a true hernia is present, fluoroscopic or ultrasonographic evaluation reveals paradoxical diaphragmatic movement caused by pressure changes during ventilation. Inspiration creates a higher negative intrathoracic pressure, which pulls the diaphragm upwards. It is important to distinguish between these 2 entities preoperatively because most pediatric surgeons would approach a diaphragmatic hernia through the abdomen in order to concomitantly correct the ever-present malrotation associated with these hernias.",
"Diaphragmatic eventration is best approached through a low thoracotomy, which enables adequate plication of the diaphragm and excision of the pulmonary sequestration that is present in many of these patients.",
"Figure 3.",
"Figure 4.",
"The patient in this case was admitted to the hospital and was kept without any oral intake. Broad-spectrum antibiotics were started, and an orogastric tube was passed to decompress the stomach. Intravenous fluids were given at a maintenance rate, and supplemental oxygen was administered. Once the patient was stable, he was brought to the operating suite, and the thorax was approached (Figures 3 and 4) through a right posterolateral muscle-sparing thoracotomy (muscle-sparing approaches diminish the incidence of postoperative scoliosis). The diaphragm was plicated with nonabsorbable sutures, without opening it (Figures 5 and 6), and the chest was closed in a regular fashion. The patient recovered uneventfully. He was extubated in the operating room and resumed diet the same day. A chest tube was not inserted, as there was no lung injury during the procedure; instead, the pleural space was drained with suction during chest closure. The patient was discharged on postoperative day 2 without respiratory difficulty.",
"Figure 5.",
"Figure 6.",
"At follow-up 6 months after surgery, the patient was doing well, was asymptomatic, and was growing and gaining weight properly. No signs of respiratory difficulty or increased incidence of respiratory infections are noted. The surgical wound had healed properly, and no scoliosis had developed. The follow-up chest radiography revealed a partially flattened diaphragm (which is a normal postoperative finding) as well as complete lung expansion."
],
"date": "June 23, 2016",
"figures": [
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/720/939/720939-thumb3.png"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/720/939/720939-thumb4.png"
},
{
"caption": "Figure 5.",
"image_url": "https://img.medscapestatic.com/article/720/939/720939-thumb5.png"
},
{
"caption": "Figure 6.",
"image_url": "https://img.medscapestatic.com/article/720/939/720939-thumb6.png"
}
],
"markdown": "# Dyspnea With Intermittent Cyanosis in a Neonate\n\n **Authors:** Jaime Shalkow, MD \n **Date:** June 23, 2016\n\n ## Content\n\n Treatment of diaphragmatic eventrations will vary, mostly according to the size of the eventration and the clinical status of the patient. Small eventrations can be left untreated, but large ones require repair, even when asymptomatic, in order to allow for proper lung development. Untreated diaphragmatic dysfunction causes a restrictive deficit with reduced lung volumes, smaller tidal volumes, and increased work of breathing. Patients with respiratory distress need prompt attention and supportive care with endotracheal intubation and ventilation.\nPatients should not have oral intake. A nasogastric tube should be passed to decompress the stomach, and intravenous fluids should be initiated. Once the patient is stable, surgery is indicated. Repair can be done through the abdomen or the chest; depending on the experience of the surgeon, the approach may be open or minimally invasive.[6] Robotic repair has been shown to be safe and effective.[7] A thoracotomy or thoracoscopy is usually preferred, except when concomitant abdominal abnormalities, such as malrotation, are suspected.\nMultiple nonabsorbable sutures are used in a staggered fashion across the diaphragm to pull and flatten the muscle. This plication maximizes the intrathoracic space by imbricating the diaphragm to a near horizontal position. Generally, chest tubes are maintained for up to several days and rapid clinical improvement is observed; however, other techniques, such as evacuation of air while closing the intercostal space, have also been described.\nThe differential diagnosis of diaphragmatic eventration includes congenital diaphragmatic hernias (also known as Bochdalek hernias). These hernias occur most commonly on the left side (90%), and the diaphragm itself is not normally visualized in radiographic examinations (except when a thick hernial sac is present).[1,2] Diaphragmatic eventrations can occur on either side of the chest, and patients are usually asymptomatic for longer periods of time because they lack the pulmonary hypoplasia associated with diaphragmatic hernias.[2] When a true hernia is present, fluoroscopic or ultrasonographic evaluation reveals paradoxical diaphragmatic movement caused by pressure changes during ventilation. Inspiration creates a higher negative intrathoracic pressure, which pulls the diaphragm upwards. It is important to distinguish between these 2 entities preoperatively because most pediatric surgeons would approach a diaphragmatic hernia through the abdomen in order to concomitantly correct the ever-present malrotation associated with these hernias.\nDiaphragmatic eventration is best approached through a low thoracotomy, which enables adequate plication of the diaphragm and excision of the pulmonary sequestration that is present in many of these patients.\nFigure 3.\nFigure 4.\nThe patient in this case was admitted to the hospital and was kept without any oral intake. Broad-spectrum antibiotics were started, and an orogastric tube was passed to decompress the stomach. Intravenous fluids were given at a maintenance rate, and supplemental oxygen was administered. Once the patient was stable, he was brought to the operating suite, and the thorax was approached (Figures 3 and 4) through a right posterolateral muscle-sparing thoracotomy (muscle-sparing approaches diminish the incidence of postoperative scoliosis). The diaphragm was plicated with nonabsorbable sutures, without opening it (Figures 5 and 6), and the chest was closed in a regular fashion. The patient recovered uneventfully. He was extubated in the operating room and resumed diet the same day. A chest tube was not inserted, as there was no lung injury during the procedure; instead, the pleural space was drained with suction during chest closure. The patient was discharged on postoperative day 2 without respiratory difficulty.\nFigure 5.\nFigure 6.\nAt follow-up 6 months after surgery, the patient was doing well, was asymptomatic, and was growing and gaining weight properly. No signs of respiratory difficulty or increased incidence of respiratory infections are noted. The surgical wound had healed properly, and no scoliosis had developed. The follow-up chest radiography revealed a partially flattened diaphragm (which is a normal postoperative finding) as well as complete lung expansion.\n\n ## Figures\n\n **Figure 3.** \n \n\n**Figure 4.** \n \n\n**Figure 5.** \n \n\n**Figure 6.** \n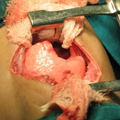 \n\n\n*Page 4 of 5*",
"pagination": {
"current_page": 4,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 340095,
"choiceText": "Ultrasonography or fluoroscopy",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 340096,
"choiceText": "MRI",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 340097,
"choiceText": "CT scan",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 340098,
"choiceText": "Lateral decubitus plain radiographs",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The criterion standard for making the diagnosis of diaphragmatic eventration is confirmation of a motionless or paradoxically moving hemidiaphragm, which can be done with fluoroscopy or with real-time ultrasonography. Ultrasonography has the advantage of not exposing the patient to ionizing radiation.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 95821,
"questionText": "A 5-day-old child is brought to the ED with wheezing, difficulty feeding, and episodic cyanosis. On examination, you notice that the patient's abdomen appears scaphoid, and a chest x-ray demonstrates bowel loops in the right hemithorax. Which of the following examinations is best for confirming the diagnosis of diaphragmatic eventration?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 340099,
"choiceText": "Bowel loops in the chest",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 340100,
"choiceText": "Free air under the diaphragm",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 340101,
"choiceText": "A partially flattened hemidiaphragm",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 340102,
"choiceText": "Infiltrate",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A partially flattened hemidiaphragm is a normal postoperative finding in a patient who has undergone eventration repair. Bowel loops in the chest is a preoperative finding, and free air under the diaphragm is concerning for a perforated abdominal viscus. An infiltrate raises concern for pneumonia.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 95822,
"questionText": "You are providing routine follow-up care for an 8-month-old boy. He underwent surgical repair of a diaphragmatic eventration as a neonate. You are considering obtaining a chest x-ray for possible pneumonia. Which of the following would you expect to see as a normal postoperative finding in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Dyspnea With Intermittent Cyanosis in a Neonate"
},
{
"authors": "Jaime Shalkow, MD",
"content": [],
"date": "June 23, 2016",
"figures": [],
"markdown": "# Dyspnea With Intermittent Cyanosis in a Neonate\n\n **Authors:** Jaime Shalkow, MD \n **Date:** June 23, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 5 of 5*",
"pagination": {
"current_page": 5,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 340095,
"choiceText": "Ultrasonography or fluoroscopy",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 340096,
"choiceText": "MRI",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 340097,
"choiceText": "CT scan",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 340098,
"choiceText": "Lateral decubitus plain radiographs",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The criterion standard for making the diagnosis of diaphragmatic eventration is confirmation of a motionless or paradoxically moving hemidiaphragm, which can be done with fluoroscopy or with real-time ultrasonography. Ultrasonography has the advantage of not exposing the patient to ionizing radiation.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 95821,
"questionText": "A 5-day-old child is brought to the ED with wheezing, difficulty feeding, and episodic cyanosis. On examination, you notice that the patient's abdomen appears scaphoid, and a chest x-ray demonstrates bowel loops in the right hemithorax. Which of the following examinations is best for confirming the diagnosis of diaphragmatic eventration?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 340099,
"choiceText": "Bowel loops in the chest",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 340100,
"choiceText": "Free air under the diaphragm",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 340101,
"choiceText": "A partially flattened hemidiaphragm",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 340102,
"choiceText": "Infiltrate",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A partially flattened hemidiaphragm is a normal postoperative finding in a patient who has undergone eventration repair. Bowel loops in the chest is a preoperative finding, and free air under the diaphragm is concerning for a perforated abdominal viscus. An infiltrate raises concern for pneumonia.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 95822,
"questionText": "You are providing routine follow-up care for an 8-month-old boy. He underwent surgical repair of a diaphragmatic eventration as a neonate. You are considering obtaining a chest x-ray for possible pneumonia. Which of the following would you expect to see as a normal postoperative finding in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Dyspnea With Intermittent Cyanosis in a Neonate"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 340091,
"choiceText": "Pericardial cyst",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 340092,
"choiceText": "Bochdalek hernia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 340093,
"choiceText": "Traumatic diaphragmatic rupture",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 340094,
"choiceText": "Diaphragmatic eventration",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 95820,
"questionText": "What is the reason for this neonate's respiratory distress?<br/>\r\n<br/>\r\n<em>Hint: Pay attention to the chest examination and look closely at the right diaphragm and hemithorax.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 340095,
"choiceText": "Ultrasonography or fluoroscopy",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 340096,
"choiceText": "MRI",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 340097,
"choiceText": "CT scan",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 340098,
"choiceText": "Lateral decubitus plain radiographs",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The criterion standard for making the diagnosis of diaphragmatic eventration is confirmation of a motionless or paradoxically moving hemidiaphragm, which can be done with fluoroscopy or with real-time ultrasonography. Ultrasonography has the advantage of not exposing the patient to ionizing radiation.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 95821,
"questionText": "A 5-day-old child is brought to the ED with wheezing, difficulty feeding, and episodic cyanosis. On examination, you notice that the patient's abdomen appears scaphoid, and a chest x-ray demonstrates bowel loops in the right hemithorax. Which of the following examinations is best for confirming the diagnosis of diaphragmatic eventration?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 340099,
"choiceText": "Bowel loops in the chest",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 340100,
"choiceText": "Free air under the diaphragm",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 340101,
"choiceText": "A partially flattened hemidiaphragm",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 340102,
"choiceText": "Infiltrate",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A partially flattened hemidiaphragm is a normal postoperative finding in a patient who has undergone eventration repair. Bowel loops in the chest is a preoperative finding, and free air under the diaphragm is concerning for a perforated abdominal viscus. An infiltrate raises concern for pneumonia.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 95822,
"questionText": "You are providing routine follow-up care for an 8-month-old boy. He underwent surgical repair of a diaphragmatic eventration as a neonate. You are considering obtaining a chest x-ray for possible pneumonia. Which of the following would you expect to see as a normal postoperative finding in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
719755 | /viewarticle/719755 | [
{
"authors": "Twinkle R. Chandak, MD",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 61-year-old man presents to the emergency department (ED) with fever, dyspnea and a productive cough for 1 week that has failed to respond to outpatient antibiotics (levofloxacin). He also complains of worsening arthralgias in both lower extremities, particularly in his knees and ankles, as well as a 10-lb (4.54-kg) weight loss over the preceding 2 months. He is a former smoker with an 80-pack-year history. No other significant medical history is noted. He denies any recent travel, sick contacts, or occupational exposure to asbestos or mineral dust. His only medications include over-the-counter analgesics for joint pains, and he denies having any drug allergies."
],
"date": "September 12, 2016",
"figures": [],
"markdown": "# A Patient With Pneumonia and Arthralgias\n\n **Authors:** Twinkle R. Chandak, MD \n **Date:** September 12, 2016\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 61-year-old man presents to the emergency department (ED) with fever, dyspnea and a productive cough for 1 week that has failed to respond to outpatient antibiotics (levofloxacin). He also complains of worsening arthralgias in both lower extremities, particularly in his knees and ankles, as well as a 10-lb (4.54-kg) weight loss over the preceding 2 months. He is a former smoker with an 80-pack-year history. No other significant medical history is noted. He denies any recent travel, sick contacts, or occupational exposure to asbestos or mineral dust. His only medications include over-the-counter analgesics for joint pains, and he denies having any drug allergies.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A Patient With Pneumonia and Arthralgias"
},
{
"authors": "Twinkle R. Chandak, MD",
"content": [
"Figure 1.",
"CT scan of the chest with IV contrast from a 61-year-old male with fever, cough, and arthralgias demonstrating a right pleural effusion with mediastinal lymphadenopathy.",
"CT scan of the chest with IV contrast from a 61-year-old male with fever, cough, and arthralgias demonstrating a right pleural effusion with mediastinal lymphadenopathy.",
"Figure 2.",
"CT scan of the chest with IV contrast from a 61-year-old male with fever, cough, and arthralgias demonstrating a right pleural effusion with lymphangitic tumor in the right lower and right middle lobes.",
"CT scan of the chest with IV contrast from a 61-year-old male with fever, cough, and arthralgias demonstrating a right pleural effusion with lymphangitic tumor in the right lower and right middle lobes.",
"The physical examination reveals an elderly, cachectic male who appears to be in mild respiratory distress. His vital signs demonstrate an oral temperature of 101.4° F (38.6º C), pulse of 100 beats/min, blood pressure of 110/70 mm Hg, respirations of 26 breaths/min, and an oxygen saturation of 93% on room air. Auscultation of the lungs demonstrates decreased breath sounds in the right lung base, with scattered fine rales. His heart sounds are regular and without any murmurs, rubs, or gallops. Abdominal examination does not reveal any tenderness or masses. Clubbing of the digits is noted; however, no evidence suggests pedal edema, joint swelling, erythema, or joint tenderness. No skin rashes are noted.",
"Laboratory tests are significant for a leukocyte count of 14 × 103/µL (14.0 × 109/L; normal range, 3.5-12.5 × 103/µL); the remainder of the laboratory findings, including hematocrit level, platelet count, electrolyte level, creatinine level, and serum glucose level, are within normal limits. A chest radiograph reveals a right lower-lobe (RLL) consolidation. The patient is started on intravenous (IV) ceftriaxone and azithromycin, and he is admitted with a diagnosis of community-acquired pneumonia.",
"Following admission, no improvement is observed in his symptoms despite antibiotics, and blood cultures, sputum cultures, and legionella serology tests return negative. A CT scan of the chest is obtained, which reveals emphysema as well as mediastinal lymphadenopathy in the pretracheal and subcarinal areas (see Figure 1). A moderate right-sided pleural effusion along with multiple subcentimeter nodular opacities in the right middle and lower lobe are also noted, along with septal thickening (see Figure 2).",
"A rheumatology consultation is obtained for his lower-extremity arthralgias. No clinical evidence suggests synovitis or effusion in any of his joints, although mild arthritis of the knees is evident, with suprapatellar enthesopathy seen on knee and ankle radiographs. He experiences no relief of his arthralgias with nonsteroidal anti-inflammatory drugs (NSAIDs) or acetaminophen. Opiates and gabapentin are added for pain relief. Additional laboratory tests are performed, which reveal an elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). Rheumatologic serology findings were negative, including rheumatoid factor, antinuclear antibody (ANA), and antineutrophil cytoplasmic antibodies (ANCAs), as well as normal serum complements. Based on the above evaluation, a presumptive diagnosis is made."
],
"date": "September 12, 2016",
"figures": [
{
"caption": "Figure 1.CT scan of the chest with IV contrast from a 61-year-old male with fever, cough, and arthralgias demonstrating a right pleural effusion with mediastinal lymphadenopathy.",
"image_url": "https://img.medscapestatic.com/article/719/755/719755-thumb1.png"
},
{
"caption": "Figure 2.CT scan of the chest with IV contrast from a 61-year-old male with fever, cough, and arthralgias demonstrating a right pleural effusion with lymphangitic tumor in the right lower and right middle lobes.",
"image_url": "https://img.medscapestatic.com/article/719/755/719755-thumb2.png"
}
],
"markdown": "# A Patient With Pneumonia and Arthralgias\n\n **Authors:** Twinkle R. Chandak, MD \n **Date:** September 12, 2016\n\n ## Content\n\n Figure 1.\nCT scan of the chest with IV contrast from a 61-year-old male with fever, cough, and arthralgias demonstrating a right pleural effusion with mediastinal lymphadenopathy.\nCT scan of the chest with IV contrast from a 61-year-old male with fever, cough, and arthralgias demonstrating a right pleural effusion with mediastinal lymphadenopathy.\nFigure 2.\nCT scan of the chest with IV contrast from a 61-year-old male with fever, cough, and arthralgias demonstrating a right pleural effusion with lymphangitic tumor in the right lower and right middle lobes.\nCT scan of the chest with IV contrast from a 61-year-old male with fever, cough, and arthralgias demonstrating a right pleural effusion with lymphangitic tumor in the right lower and right middle lobes.\nThe physical examination reveals an elderly, cachectic male who appears to be in mild respiratory distress. His vital signs demonstrate an oral temperature of 101.4° F (38.6º C), pulse of 100 beats/min, blood pressure of 110/70 mm Hg, respirations of 26 breaths/min, and an oxygen saturation of 93% on room air. Auscultation of the lungs demonstrates decreased breath sounds in the right lung base, with scattered fine rales. His heart sounds are regular and without any murmurs, rubs, or gallops. Abdominal examination does not reveal any tenderness or masses. Clubbing of the digits is noted; however, no evidence suggests pedal edema, joint swelling, erythema, or joint tenderness. No skin rashes are noted.\nLaboratory tests are significant for a leukocyte count of 14 × 103/µL (14.0 × 109/L; normal range, 3.5-12.5 × 103/µL); the remainder of the laboratory findings, including hematocrit level, platelet count, electrolyte level, creatinine level, and serum glucose level, are within normal limits. A chest radiograph reveals a right lower-lobe (RLL) consolidation. The patient is started on intravenous (IV) ceftriaxone and azithromycin, and he is admitted with a diagnosis of community-acquired pneumonia.\nFollowing admission, no improvement is observed in his symptoms despite antibiotics, and blood cultures, sputum cultures, and legionella serology tests return negative. A CT scan of the chest is obtained, which reveals emphysema as well as mediastinal lymphadenopathy in the pretracheal and subcarinal areas (see Figure 1). A moderate right-sided pleural effusion along with multiple subcentimeter nodular opacities in the right middle and lower lobe are also noted, along with septal thickening (see Figure 2).\nA rheumatology consultation is obtained for his lower-extremity arthralgias. No clinical evidence suggests synovitis or effusion in any of his joints, although mild arthritis of the knees is evident, with suprapatellar enthesopathy seen on knee and ankle radiographs. He experiences no relief of his arthralgias with nonsteroidal anti-inflammatory drugs (NSAIDs) or acetaminophen. Opiates and gabapentin are added for pain relief. Additional laboratory tests are performed, which reveal an elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). Rheumatologic serology findings were negative, including rheumatoid factor, antinuclear antibody (ANA), and antineutrophil cytoplasmic antibodies (ANCAs), as well as normal serum complements. Based on the above evaluation, a presumptive diagnosis is made.\n\n ## Figures\n\n **Figure 1.CT scan of the chest with IV contrast from a 61-year-old male with fever, cough, and arthralgias demonstrating a right pleural effusion with mediastinal lymphadenopathy.** \n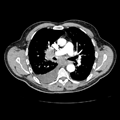 \n\n**Figure 2.CT scan of the chest with IV contrast from a 61-year-old male with fever, cough, and arthralgias demonstrating a right pleural effusion with lymphangitic tumor in the right lower and right middle lobes.** \n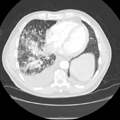 \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 336487,
"choiceText": "<em>Mycoplasma pneumoniae</em>",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336488,
"choiceText": "Rheumatic fever",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336489,
"choiceText": "Septic emboli from culture-negative endocarditis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336490,
"choiceText": "Hypertrophic osteoarthropathy",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 94525,
"questionText": "What is the most likely diagnosis?<br/>\r\n<br/>\r\n<em>Hint: Review the chest CT scan.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Patient With Pneumonia and Arthralgias"
},
{
"authors": "Twinkle R. Chandak, MD",
"content": [
"Figure 3.",
"Bone scintigraphy showing irregular uptake but no metastatic disease.",
"Bone scintigraphy showing irregular uptake but no metastatic disease.",
"Based on the CT scan, the patient was suspected to have malignancy and an associated hypertrophic osteoarthropathy (HOA). After obtaining the studies above, the patient underwent mediastinoscopy and lymph node biopsy, which resulted in the diagnosis of poorly differentiated non–small cell lung cancer (NSCLC). Thoracentesis confirmed the malignant nature of the pleural fluid. Bone scintigraphy showed no metastatic disease, but it did demonstrate irregular uptake in both tibiae and fibulae, with evidence of arthritis in the major joints (see Figure 3); these findings are consistent with the final diagnosis of NSCLC-associated HOA.",
"HOA syndrome is a condition characterized by proliferative periostitis of the long bones, especially in the distal and periarticular regions. HOA can result in proliferation of the synovial membranes, which causes painful and swollen joints; it is often accompanied by clubbing of the digits.[1,2] Although clubbing was first described by Hippocrates in the 5th century BC,[2] the association of clubbing, arthralgia, and ossifying periostitis as a distinct clinical syndrome was not recognized until 1889 and 1890 by Bamberger[3] and Marie,[4] respectively. HOA is also known as the Pierre Marie-Bamberger syndrome.",
"This syndrome is classified into primary and secondary HOA. Primary HOA is not associated with any other medical disorders; however, secondary HOA (which is more common) is generally associated with lung cancer, tuberculosis, pulmonary abscess, bronchiectasis, emphysema, cystic fibrosis, interstitial lung disease, right-to-left cardiac shunts, and, less often, other disorders (eg, Hodgkin lymphoma and cirrhosis).[1,5,6] Primary or idiopathic HOA (also called pachydermoperiostosis and Touraine-Solente-Gole syndrome) is a hereditary disorder that presents in childhood and clinically mimics secondary HOA. It may be associated with bilateral eyelid ptosis, leonine facies, and thickened skin. The genetic abnormality in primary HOA involves a mutation in the hydroxyprostaglandin dehydrogenase (HPGD) gene that encodes 15-hydroxyprostaglandin dehydrogenase, which is the primary enzyme responsible for prostaglandin degradation.[7]",
"The incidence of secondary HOA in patients diagnosed with lung cancer, either primary or metastatic, is approximately 4-5%. Approximately 80% of pulmonary lesions associated with HOA are lung cancers; pleural tumors account for 10%, and a miscellaneous group of intrathoracic malignancies account for 5%.[8] Among patients with lung cancer, HOA is associated with all cell types, most frequently with adenocarcinoma and less frequently with small cell carcinoma.[1] This secondary form of HOA is also known as hypertrophic pulmonary osteoarthropathy (HPOA).",
"Although the etiology of HOA is still poorly understood, both neurogenic and humoral mechanisms may play a role. Clubbing and HOA appear to be different manifestations of the same disease process.[9] Localized activation of platelet-endothelial cells, with the subsequent release of fibroblast growth factors (eg, platelet-derived growth factor, PDGF) is thought to play an important role in the pathogenesis of HOA. The frequent association of HOA with lung disease raises the possibility that circulatory bypass of the lungs may be responsible. One hypothesis regarding the etiology of HOA theorizes that megakaryocytes escape their normal fragmentation to platelets in the lung and reach the distal extremities, where they release growth factors.[10,11]",
"Another hypothesis involves tumor production and the release of factors into the circulation (eg, vascular endothelial growth factor [VEGF]) that promote features of HOA, such as vascular proliferation, edema formation, and new bone formation. Two case reports of patients with lung cancer and HOA reported elevated circulating concentrations of VEGF.[10,12] In one case, a marked decline in VEGF followed resection of tumor and was temporally correlated with the disappearance of the skeletal abnormalities.[12] Elevated levels of PDGF, endothelin-1 (ET-1), β-thromboglobulin (β-TG), and VEGF have all been reported in patients with HOA.[11] Estrogen and growth hormone (GH) produced by pulmonary tumors has also been implicated in the development of HOA. In one case report, previously high levels of GH were noted to return to normal after resection of tumor, along with relief of clinical symptoms.[8] Finally, the central abnormality in primary HOA, prostaglandin E2, may also play a pathogenetic role in secondary HOA.[7]"
],
"date": "September 12, 2016",
"figures": [
{
"caption": "Figure 3.Bone scintigraphy showing irregular uptake but no metastatic disease.",
"image_url": "https://img.medscapestatic.com/article/719/755/719755-thumb3.png"
}
],
"markdown": "# A Patient With Pneumonia and Arthralgias\n\n **Authors:** Twinkle R. Chandak, MD \n **Date:** September 12, 2016\n\n ## Content\n\n Figure 3.\nBone scintigraphy showing irregular uptake but no metastatic disease.\nBone scintigraphy showing irregular uptake but no metastatic disease.\nBased on the CT scan, the patient was suspected to have malignancy and an associated hypertrophic osteoarthropathy (HOA). After obtaining the studies above, the patient underwent mediastinoscopy and lymph node biopsy, which resulted in the diagnosis of poorly differentiated non–small cell lung cancer (NSCLC). Thoracentesis confirmed the malignant nature of the pleural fluid. Bone scintigraphy showed no metastatic disease, but it did demonstrate irregular uptake in both tibiae and fibulae, with evidence of arthritis in the major joints (see Figure 3); these findings are consistent with the final diagnosis of NSCLC-associated HOA.\nHOA syndrome is a condition characterized by proliferative periostitis of the long bones, especially in the distal and periarticular regions. HOA can result in proliferation of the synovial membranes, which causes painful and swollen joints; it is often accompanied by clubbing of the digits.[1,2] Although clubbing was first described by Hippocrates in the 5th century BC,[2] the association of clubbing, arthralgia, and ossifying periostitis as a distinct clinical syndrome was not recognized until 1889 and 1890 by Bamberger[3] and Marie,[4] respectively. HOA is also known as the Pierre Marie-Bamberger syndrome.\nThis syndrome is classified into primary and secondary HOA. Primary HOA is not associated with any other medical disorders; however, secondary HOA (which is more common) is generally associated with lung cancer, tuberculosis, pulmonary abscess, bronchiectasis, emphysema, cystic fibrosis, interstitial lung disease, right-to-left cardiac shunts, and, less often, other disorders (eg, Hodgkin lymphoma and cirrhosis).[1,5,6] Primary or idiopathic HOA (also called pachydermoperiostosis and Touraine-Solente-Gole syndrome) is a hereditary disorder that presents in childhood and clinically mimics secondary HOA. It may be associated with bilateral eyelid ptosis, leonine facies, and thickened skin. The genetic abnormality in primary HOA involves a mutation in the hydroxyprostaglandin dehydrogenase (HPGD) gene that encodes 15-hydroxyprostaglandin dehydrogenase, which is the primary enzyme responsible for prostaglandin degradation.[7]\nThe incidence of secondary HOA in patients diagnosed with lung cancer, either primary or metastatic, is approximately 4-5%. Approximately 80% of pulmonary lesions associated with HOA are lung cancers; pleural tumors account for 10%, and a miscellaneous group of intrathoracic malignancies account for 5%.[8] Among patients with lung cancer, HOA is associated with all cell types, most frequently with adenocarcinoma and less frequently with small cell carcinoma.[1] This secondary form of HOA is also known as hypertrophic pulmonary osteoarthropathy (HPOA).\nAlthough the etiology of HOA is still poorly understood, both neurogenic and humoral mechanisms may play a role. Clubbing and HOA appear to be different manifestations of the same disease process.[9] Localized activation of platelet-endothelial cells, with the subsequent release of fibroblast growth factors (eg, platelet-derived growth factor, PDGF) is thought to play an important role in the pathogenesis of HOA. The frequent association of HOA with lung disease raises the possibility that circulatory bypass of the lungs may be responsible. One hypothesis regarding the etiology of HOA theorizes that megakaryocytes escape their normal fragmentation to platelets in the lung and reach the distal extremities, where they release growth factors.[10,11]\nAnother hypothesis involves tumor production and the release of factors into the circulation (eg, vascular endothelial growth factor [VEGF]) that promote features of HOA, such as vascular proliferation, edema formation, and new bone formation. Two case reports of patients with lung cancer and HOA reported elevated circulating concentrations of VEGF.[10,12] In one case, a marked decline in VEGF followed resection of tumor and was temporally correlated with the disappearance of the skeletal abnormalities.[12] Elevated levels of PDGF, endothelin-1 (ET-1), β-thromboglobulin (β-TG), and VEGF have all been reported in patients with HOA.[11] Estrogen and growth hormone (GH) produced by pulmonary tumors has also been implicated in the development of HOA. In one case report, previously high levels of GH were noted to return to normal after resection of tumor, along with relief of clinical symptoms.[8] Finally, the central abnormality in primary HOA, prostaglandin E2, may also play a pathogenetic role in secondary HOA.[7]\n\n ## Figures\n\n **Figure 3.Bone scintigraphy showing irregular uptake but no metastatic disease.** \n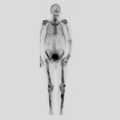 \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 336487,
"choiceText": "<em>Mycoplasma pneumoniae</em>",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336488,
"choiceText": "Rheumatic fever",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336489,
"choiceText": "Septic emboli from culture-negative endocarditis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336490,
"choiceText": "Hypertrophic osteoarthropathy",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 94525,
"questionText": "What is the most likely diagnosis?<br/>\r\n<br/>\r\n<em>Hint: Review the chest CT scan.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Patient With Pneumonia and Arthralgias"
},
{
"authors": "Twinkle R. Chandak, MD",
"content": [
"The clinical features of HOA include digital clubbing and periostitis or periosteal new bone formation of tubular bones (particularly the long bones of the distal extremities). Periostitis causes severe burning pain in the distal extremities that is aggravated by dependency and weight bearing. It is usually accompanied by tenderness to palpation of the involved areas.",
"Some patients present with a painful, symmetric, arthritislike changes in the large joints and periarticular tissues (ankles, knees, wrists, metacarpopharyngeal joints, and elbows).[13] HOA mimics rheumatoid arthritis clinically; however, synovial fluids have been reported to be typically noninflammatory, with leukocyte counts less than 0.5 × 103/µL (0.5 × 109/L). The synovial membrane in cases of HOA demonstrates vascular congestion with mild lymphocytic infiltration.[13] Increased thickness of the subcutaneous soft tissues may be noted in the distal one-third of the arms and legs and, sometimes, of the facial tissues, which may simulate acromegaly. Neurovascular changes of the hands and feet, including chronic erythema, paresthesia, and increased sweating, may occur.",
"In primary HOA, bone and joint pain tends to be less severe, whereas furrowing of the face and scalp tends to be more severe.[7] Patients with full-blown HOA may in fact initially present to rheumatologists with severe arthritic symptoms, or they may even see a cardiologist with bilateral ankle swelling presumed to be caused by congestive heart failure.",
"When HOA is suspected, diagnostic tests should be directed at the chest because the most frequent cause of acute-onset HOA is a primary or secondary lung neoplasm. In one-third of patients with lung cancer, clinical HOA predates the onset of respiratory symptoms, whereas in another third, patients present with respiratory symptoms simultaneously. In the remaining patients, signs and symptoms of HOA may appear after the diagnosis of malignancy is established.[1] Removal of lung cancer or the treatment of other causes of HOA results in regression of the clinical manifestations of HOA.[1]",
"In mild-to-moderate cases, symptom control may be attempted with analgesics, such as nonsteroidal anti-inflammatory medications (NSAIDs), steroids, or narcotics; however, many patients find the associated pain to be disabling and their symptoms are often resistant to these treatments. A single dose of 4 mg of zoledronic acid has been effectively used to alleviate symptoms. In refractory cases, subcutaneous octreotide may be used to relieve symptoms.",
"Laboratory testing is not particularly useful in evaluating HOA; however, an elevated ESR of more than 50 mm/h and, in advanced cases, an elevated alkaline phosphatase level may be found.[13]",
"Imaging studies are important in evaluating HOA. Radionuclide studies are more sensitive than radiography in the detection of HOA. The appearance of HOA on radionuclide studies can range from an increased \"braceletlike\" appearance to more diffuse, symmetrically increased uptake along the cortical margins of the diaphyses of the long, tubular bones (sometimes referred to as the \"parallel tract\" or \"double stripe sign\"). Although uncommon, asymmetric and irregular involvement of the long bones (as seen in this case) may be noted. Increased uptake in the distal phalanges is associated with marked clubbing. Although usually located in the peripheral skeleton, HOA can also affect the skull, clavicles, ribs, and scapulae. The disease is typically more active in the lower extremities than in the upper ones, and it is usually greater in the long bones distal to the knees and elbows than those proximal to these joints."
],
"date": "September 12, 2016",
"figures": [],
"markdown": "# A Patient With Pneumonia and Arthralgias\n\n **Authors:** Twinkle R. Chandak, MD \n **Date:** September 12, 2016\n\n ## Content\n\n The clinical features of HOA include digital clubbing and periostitis or periosteal new bone formation of tubular bones (particularly the long bones of the distal extremities). Periostitis causes severe burning pain in the distal extremities that is aggravated by dependency and weight bearing. It is usually accompanied by tenderness to palpation of the involved areas.\nSome patients present with a painful, symmetric, arthritislike changes in the large joints and periarticular tissues (ankles, knees, wrists, metacarpopharyngeal joints, and elbows).[13] HOA mimics rheumatoid arthritis clinically; however, synovial fluids have been reported to be typically noninflammatory, with leukocyte counts less than 0.5 × 103/µL (0.5 × 109/L). The synovial membrane in cases of HOA demonstrates vascular congestion with mild lymphocytic infiltration.[13] Increased thickness of the subcutaneous soft tissues may be noted in the distal one-third of the arms and legs and, sometimes, of the facial tissues, which may simulate acromegaly. Neurovascular changes of the hands and feet, including chronic erythema, paresthesia, and increased sweating, may occur.\nIn primary HOA, bone and joint pain tends to be less severe, whereas furrowing of the face and scalp tends to be more severe.[7] Patients with full-blown HOA may in fact initially present to rheumatologists with severe arthritic symptoms, or they may even see a cardiologist with bilateral ankle swelling presumed to be caused by congestive heart failure.\nWhen HOA is suspected, diagnostic tests should be directed at the chest because the most frequent cause of acute-onset HOA is a primary or secondary lung neoplasm. In one-third of patients with lung cancer, clinical HOA predates the onset of respiratory symptoms, whereas in another third, patients present with respiratory symptoms simultaneously. In the remaining patients, signs and symptoms of HOA may appear after the diagnosis of malignancy is established.[1] Removal of lung cancer or the treatment of other causes of HOA results in regression of the clinical manifestations of HOA.[1]\nIn mild-to-moderate cases, symptom control may be attempted with analgesics, such as nonsteroidal anti-inflammatory medications (NSAIDs), steroids, or narcotics; however, many patients find the associated pain to be disabling and their symptoms are often resistant to these treatments. A single dose of 4 mg of zoledronic acid has been effectively used to alleviate symptoms. In refractory cases, subcutaneous octreotide may be used to relieve symptoms.\nLaboratory testing is not particularly useful in evaluating HOA; however, an elevated ESR of more than 50 mm/h and, in advanced cases, an elevated alkaline phosphatase level may be found.[13]\nImaging studies are important in evaluating HOA. Radionuclide studies are more sensitive than radiography in the detection of HOA. The appearance of HOA on radionuclide studies can range from an increased \"braceletlike\" appearance to more diffuse, symmetrically increased uptake along the cortical margins of the diaphyses of the long, tubular bones (sometimes referred to as the \"parallel tract\" or \"double stripe sign\"). Although uncommon, asymmetric and irregular involvement of the long bones (as seen in this case) may be noted. Increased uptake in the distal phalanges is associated with marked clubbing. Although usually located in the peripheral skeleton, HOA can also affect the skull, clavicles, ribs, and scapulae. The disease is typically more active in the lower extremities than in the upper ones, and it is usually greater in the long bones distal to the knees and elbows than those proximal to these joints.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A Patient With Pneumonia and Arthralgias"
},
{
"authors": "Twinkle R. Chandak, MD",
"content": [
"The scintigraphic abnormalities found in the peripheral skeleton of patients with HOA are not easily mistaken for diffuse skeletal metastasis. Metastatic tumor almost always involves the central skeleton in an irregular, focal, asymmetric pattern. When long bones are involved in cases of metastasis, it is the medullary cavity that is primarily affected, as opposed to the cortical involvement seen in HOA. The radiographic and radionuclide image findings may diminish or even disappear following appropriate therapy of the associated disease process.",
"HOA has no prognostic significance and early detection may lead to the discovery of a potentially resectable lung carcinoma. Subclinical cases may be diagnosed by radiographs or by skeletal scintigraphy (which is more sensitive than plain radiographs), with an incidence of lung cancer of approximately 20%.",
"HOA should be considered in the differential diagnosis of bone and joint pains in patients with cancer in addition to bone metastasis or inflammatory arthritides. Clinicians should be aware of the clinical entity of HOA and the radiographic findings of periostitis, which may lead to early detection of lung cancer in patients without significant pulmonary symptoms and avoidance of possible tumor progression and distant metastases.",
"The patient in this case began chemotherapy treatment for his NSCLC. The chemotherapy improved his arthralgias significantly. He was later discharged with outpatient oncology follow-up. After discharge, the patient did very well with high-dose NSAIDS and opiates, and he followed up with the oncologist for further treatment of his lung cancer."
],
"date": "September 12, 2016",
"figures": [],
"markdown": "# A Patient With Pneumonia and Arthralgias\n\n **Authors:** Twinkle R. Chandak, MD \n **Date:** September 12, 2016\n\n ## Content\n\n The scintigraphic abnormalities found in the peripheral skeleton of patients with HOA are not easily mistaken for diffuse skeletal metastasis. Metastatic tumor almost always involves the central skeleton in an irregular, focal, asymmetric pattern. When long bones are involved in cases of metastasis, it is the medullary cavity that is primarily affected, as opposed to the cortical involvement seen in HOA. The radiographic and radionuclide image findings may diminish or even disappear following appropriate therapy of the associated disease process.\nHOA has no prognostic significance and early detection may lead to the discovery of a potentially resectable lung carcinoma. Subclinical cases may be diagnosed by radiographs or by skeletal scintigraphy (which is more sensitive than plain radiographs), with an incidence of lung cancer of approximately 20%.\nHOA should be considered in the differential diagnosis of bone and joint pains in patients with cancer in addition to bone metastasis or inflammatory arthritides. Clinicians should be aware of the clinical entity of HOA and the radiographic findings of periostitis, which may lead to early detection of lung cancer in patients without significant pulmonary symptoms and avoidance of possible tumor progression and distant metastases.\nThe patient in this case began chemotherapy treatment for his NSCLC. The chemotherapy improved his arthralgias significantly. He was later discharged with outpatient oncology follow-up. After discharge, the patient did very well with high-dose NSAIDS and opiates, and he followed up with the oncologist for further treatment of his lung cancer.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 336491,
"choiceText": "Elevated troponin",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336492,
"choiceText": "Synovial fluid leukocyte counts of greater than 0.5 × 10<sup>3</sup>/µL",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336493,
"choiceText": "Elevated erythrocyte sedimentation rate",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336494,
"choiceText": "Low alkaline phosphatase level",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Laboratory findings often reveal an elevated ESR of more than 50 mm/h and, in advanced cases, an elevated alkaline phosphatase level can be found.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 94526,
"questionText": "You are caring for a patient with fevers, cough, and arthralgias, and you are concerned that this patient may have HOA. You order laboratory studies. Though nonspecific, which laboratory abnormality would you most likely find in a patient with HOA?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 336495,
"choiceText": "CT scanning",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336496,
"choiceText": "Conventional radiography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336497,
"choiceText": "MRI",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336498,
"choiceText": "Radionuclide studies",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336499,
"choiceText": "Ultrasonography",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Radionuclide studies are more sensitive than radiography in the detection of HOA. Its appearance can range from an increased \"braceletlike\" appearance to more diffuse, symmetrically increased uptake along the cortical margins of the diaphyses of the long, tubular bones, sometimes referred to as the \"parallel tract\" or \"double stripe sign.\"",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 94527,
"questionText": "You are considering an imaging test to confirm the diagnosis of HOA in the patient above. Which of the following imaging modalities is best for diagnosing HOA in your patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Patient With Pneumonia and Arthralgias"
},
{
"authors": "Twinkle R. Chandak, MD",
"content": [],
"date": "September 12, 2016",
"figures": [],
"markdown": "# A Patient With Pneumonia and Arthralgias\n\n **Authors:** Twinkle R. Chandak, MD \n **Date:** September 12, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 336491,
"choiceText": "Elevated troponin",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336492,
"choiceText": "Synovial fluid leukocyte counts of greater than 0.5 × 10<sup>3</sup>/µL",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336493,
"choiceText": "Elevated erythrocyte sedimentation rate",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336494,
"choiceText": "Low alkaline phosphatase level",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Laboratory findings often reveal an elevated ESR of more than 50 mm/h and, in advanced cases, an elevated alkaline phosphatase level can be found.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 94526,
"questionText": "You are caring for a patient with fevers, cough, and arthralgias, and you are concerned that this patient may have HOA. You order laboratory studies. Though nonspecific, which laboratory abnormality would you most likely find in a patient with HOA?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 336495,
"choiceText": "CT scanning",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336496,
"choiceText": "Conventional radiography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336497,
"choiceText": "MRI",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336498,
"choiceText": "Radionuclide studies",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336499,
"choiceText": "Ultrasonography",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Radionuclide studies are more sensitive than radiography in the detection of HOA. Its appearance can range from an increased \"braceletlike\" appearance to more diffuse, symmetrically increased uptake along the cortical margins of the diaphyses of the long, tubular bones, sometimes referred to as the \"parallel tract\" or \"double stripe sign.\"",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 94527,
"questionText": "You are considering an imaging test to confirm the diagnosis of HOA in the patient above. Which of the following imaging modalities is best for diagnosing HOA in your patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Patient With Pneumonia and Arthralgias"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 336487,
"choiceText": "<em>Mycoplasma pneumoniae</em>",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336488,
"choiceText": "Rheumatic fever",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336489,
"choiceText": "Septic emboli from culture-negative endocarditis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336490,
"choiceText": "Hypertrophic osteoarthropathy",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 94525,
"questionText": "What is the most likely diagnosis?<br/>\r\n<br/>\r\n<em>Hint: Review the chest CT scan.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 336491,
"choiceText": "Elevated troponin",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336492,
"choiceText": "Synovial fluid leukocyte counts of greater than 0.5 × 10<sup>3</sup>/µL",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336493,
"choiceText": "Elevated erythrocyte sedimentation rate",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336494,
"choiceText": "Low alkaline phosphatase level",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Laboratory findings often reveal an elevated ESR of more than 50 mm/h and, in advanced cases, an elevated alkaline phosphatase level can be found.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 94526,
"questionText": "You are caring for a patient with fevers, cough, and arthralgias, and you are concerned that this patient may have HOA. You order laboratory studies. Though nonspecific, which laboratory abnormality would you most likely find in a patient with HOA?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 336495,
"choiceText": "CT scanning",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336496,
"choiceText": "Conventional radiography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336497,
"choiceText": "MRI",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336498,
"choiceText": "Radionuclide studies",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 336499,
"choiceText": "Ultrasonography",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Radionuclide studies are more sensitive than radiography in the detection of HOA. Its appearance can range from an increased \"braceletlike\" appearance to more diffuse, symmetrically increased uptake along the cortical margins of the diaphyses of the long, tubular bones, sometimes referred to as the \"parallel tract\" or \"double stripe sign.\"",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 94527,
"questionText": "You are considering an imaging test to confirm the diagnosis of HOA in the patient above. Which of the following imaging modalities is best for diagnosing HOA in your patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
717127 | /viewarticle/717127 | [
{
"authors": "Craig A. Goolsby, MD; Erik D. Schraga, MD",
"content": [
"Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.",
"An 8-day-old boy is brought into the emergency department (ED) by his parents for a chief complaint of rapid breathing. During the past several hours, the patient's mother noted an increased respiratory rate and decreased feeding ability. The child had previously fed for 10 minutes at each breast every 2 hours, but he is now crying and unable to latch on for more than a few seconds at each breast. Both parents state that they think the patient looks pale. Up until the day of presentation, the patient had been doing well, with no prior episodes of difficulty breathing. He was born full-term via normal spontaneous vaginal delivery, without complications during the pregnancy or delivery. He has had no sick contacts and has not been in day care. He has not had a fever, cough, or runny nose. His urine output has been normal, and he has had 2-3 nonbloody bowel movements daily. Other than spitting up a small amount after feedings, no episodes of vomiting are reported. The parents state that he seems increasingly fussy. No episodes of apnea, cyanosis, choking, or gagging have been reported, nor have any discernible changes in muscle tone.",
"Figure 1."
],
"date": "January 29, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/717/127/717127-thumb1.png"
}
],
"markdown": "# An 8-Day-Old Boy With Tachypnea and Difficulty Feeding\n\n **Authors:** Craig A. Goolsby, MD; Erik D. Schraga, MD \n **Date:** January 29, 2015\n\n ## Content\n\n Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.\nAn 8-day-old boy is brought into the emergency department (ED) by his parents for a chief complaint of rapid breathing. During the past several hours, the patient's mother noted an increased respiratory rate and decreased feeding ability. The child had previously fed for 10 minutes at each breast every 2 hours, but he is now crying and unable to latch on for more than a few seconds at each breast. Both parents state that they think the patient looks pale. Up until the day of presentation, the patient had been doing well, with no prior episodes of difficulty breathing. He was born full-term via normal spontaneous vaginal delivery, without complications during the pregnancy or delivery. He has had no sick contacts and has not been in day care. He has not had a fever, cough, or runny nose. His urine output has been normal, and he has had 2-3 nonbloody bowel movements daily. Other than spitting up a small amount after feedings, no episodes of vomiting are reported. The parents state that he seems increasingly fussy. No episodes of apnea, cyanosis, choking, or gagging have been reported, nor have any discernible changes in muscle tone.\nFigure 1.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 1 of 5*",
"pagination": {
"current_page": 1,
"total_pages": 5
},
"questionnaire": [],
"title": "An 8-Day-Old Boy With Tachypnea and Difficulty Feeding"
},
{
"authors": "Craig A. Goolsby, MD; Erik D. Schraga, MD",
"content": [
"Physical Examination and Work-up",
"On physical examination, his vital signs are significant for a respiratory rate of 70 breaths/min and a heart rate of 296 bpm. He is afebrile, with a rectal temperature of 98.2°F (36.8°C). His blood pressure is 72/40 mm Hg. His oxygen saturation, measured by pulse oximetry, is 98% while breathing room air. Despite the abnormal vital signs, the baby does not appear distressed or even uncomfortable. He is moving all extremities, and he opens his eyes and looks around. His oropharynx is clear, with no visible foreign bodies. Rhinorrhea and nasal congestion have not been observed. His lung sounds are clear, and despite the significant tachypnea, no retractions, grunting, or nasal flaring are present. On auscultation of the heart, rapid heart sounds are noted, and as a result of the tachycardia, an assessment for murmurs is not possible. His distal extremities are pink and have normal capillary refill.",
"While placing an intravenous line and connecting the child to a monitor, an electrocardiogram (ECG) is obtained."
],
"date": "January 29, 2015",
"figures": [],
"markdown": "# An 8-Day-Old Boy With Tachypnea and Difficulty Feeding\n\n **Authors:** Craig A. Goolsby, MD; Erik D. Schraga, MD \n **Date:** January 29, 2015\n\n ## Content\n\n Physical Examination and Work-up\nOn physical examination, his vital signs are significant for a respiratory rate of 70 breaths/min and a heart rate of 296 bpm. He is afebrile, with a rectal temperature of 98.2°F (36.8°C). His blood pressure is 72/40 mm Hg. His oxygen saturation, measured by pulse oximetry, is 98% while breathing room air. Despite the abnormal vital signs, the baby does not appear distressed or even uncomfortable. He is moving all extremities, and he opens his eyes and looks around. His oropharynx is clear, with no visible foreign bodies. Rhinorrhea and nasal congestion have not been observed. His lung sounds are clear, and despite the significant tachypnea, no retractions, grunting, or nasal flaring are present. On auscultation of the heart, rapid heart sounds are noted, and as a result of the tachycardia, an assessment for murmurs is not possible. His distal extremities are pink and have normal capillary refill.\nWhile placing an intravenous line and connecting the child to a monitor, an electrocardiogram (ECG) is obtained.\n\n ## Figures\n\n \n*Page 2 of 5*",
"pagination": {
"current_page": 2,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 329839,
"choiceText": "Torsade de pointes",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329840,
"choiceText": "Atrial fibrillation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329841,
"choiceText": "Supraventricular tachycardia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329842,
"choiceText": "Ventricular tachycardia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 92180,
"questionText": "What type of arrhythmia is shown on the ECG?<br/>\r\n<br/>\r\n<em>Hint: Consider the morphology of QRS complexes (narrow vs wide) and the timing of the complexes (regular vs irregular).</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 8-Day-Old Boy With Tachypnea and Difficulty Feeding"
},
{
"authors": "Craig A. Goolsby, MD; Erik D. Schraga, MD",
"content": [
"This patient was diagnosed with supraventricular tachycardia (SVT), which is defined as a regular, rapid rhythm that requires only atrial or atrioventricular tissue for its initiation and maintenance. Paroxysmal SVT (PSVT) is the most common dysrhythmia in children. It has an international prevalence of about 2 cases per 1,000 people. PSVT tends to manifest in infancy and early childhood.[4]",
"Figure 1.",
"The presentation of PSVT widely varies in children, ranging from an incidental finding in an asymptomatic patient to fulminant cardiogenic shock. Infants present with caretakers complaining of rapid breathing, poor feeding, sweating with feeding, pallor, lethargy, and excessive crying. Older children may complain of chest pain, shortness of breath, and palpitations. The physical examination is likewise variable, depending upon the child's age and heart rate and on the duration of the episode (which can last from seconds to days). For infants, the examination is remarkable for a regular tachycardia. Normal resting heart rates for neonates can be up to 160 bpm. The upper limit of the normal heart rate decreases with age until late adolescence, when children's heart rates are similar to those of adults. Heart rates ranging beyond the normal upper limits, particularly in excess of 240, should be highly suspicious for SVT. In addition to tachycardia, infants may have physical findings of pallor, irritability, lethargy, tachypnea, weight loss (or failure to gain), poor perfusion, weak pulses/hypotension, hepatomegaly, and, sometimes, cardiogenic shock. A pounding sensation in the neck may be caused by cannon A waves, which occur when the atrium contracts at the same time as the ventricle. Older children typically have benign examinations (except for the findings of tachycardia and tachypnea).[1,3,6]",
"The 3 types of SVT are (1) atrial tachycardia (ectopic, or nonreciprocating, atrial tachycardia), (2) atrioventricular nodal reentrant tachycardia (AVNRT), and (3) atrioventricular reentrant (or reciprocating) tachycardia (AVRT). In the United States, reentrant tachycardias are the most common cause of PSVT in the pediatric population, AVRT is more common than AVRT in children younger than 12 years. AVRT consists of 2 or more functionally (and, usually, anatomically) distinct pathways between the atria and ventricles. The first pathway is usually the atrioventricular (AV) node. The second is an accessory pathway that may be an anatomically separate bypass tract between the atrium and ventricle (such as the bundle of Kent). Wolff-Parkinson-White (WPW) syndrome preexcitation is a good example of SVT caused by an anatomically separate bypass tract. Each pathway has different electrophysiologic characteristics; one pathway is fast (a short conduction time and a long refractory period), while the other is slow (a longer conduction time and a shorter refractory period). In AVNRT, both pathways exist in the AV node itself. In AVNRT, for example, while the child is in regular sinus, a premature atrial beat may block in the fast pathway (because of its longer refractory period). The classification of AVRT includes distinguishing antidromic (down the accessory pathway and back up the AV node) from orthodromic (down the AV node back up the accessory pathway).",
"Because the fast pathway is blocked, the signal conducts down the slow pathway. Then, when the impulse reaches the insertion of the fast pathway, which has now recovered after its refractory period, the impulse conducts in a retrograde fashion through the fast pathway. This results in a circuit loop tachycardia, with an impulse moving in a loop down the slow pathway and up the fast one.[1,3,4,5,6] A circuit loop in which impulses go down the slow pathway and back up the fast pathway is a typical AVNRT; in atypical AVNRT, the impulse goes down the fast pathway and back up the slow pathway. On an ECG these can be differentiated by where the P wave (atrial activation) is in relation to the QRS complex.",
"Other causes of PSVT include sympathomimetic stimulation (such as medications for upper respiratory infection), structural defects, and atrial ectopy. About half of all SVT cases occur without underlying heart disease; these cases are termed idiopathic. Idiopathic SVT is more common in younger patients than in older children. WPW syndrome preexcitation accounts for 10-20% of cases. Congenital heart defects such as Ebstein anomaly may also predispose children to SVT. Children who have undergone cardiac surgery are also more prone to developing this arrhythmia.[3]",
"The diagnosis of PSVT is made based on patient history, physical examination findings, and ECG findings. The ECG findings include an excessively rapid (usually between 200 and 280 bpm) regular tachycardia, often without discernible P-waves preceding the QRS complexes. P-waves, when visible in PSVT, may have an abnormal axis and may be seen within or following the QRS complexes. In most cases, the tachycardia will be narrow complex; however, wide-complex PSVT is possible if aberrancy is present or if the AVNR conduction occurs in an antidromic fashion with WPW syndrome. It is important to distinguish PSVT from sinus tachycardia, which is the most common tachycardia in children. Sinus tachycardia and PSVT have dramatically different treatments. Laboratory evaluation depends on the clinical scenario, but practitioners should consider checking electrolytes, thyroid function, and hemoglobin level.[4]"
],
"date": "January 29, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/717/127/717127-thumb1.png"
}
],
"markdown": "# An 8-Day-Old Boy With Tachypnea and Difficulty Feeding\n\n **Authors:** Craig A. Goolsby, MD; Erik D. Schraga, MD \n **Date:** January 29, 2015\n\n ## Content\n\n This patient was diagnosed with supraventricular tachycardia (SVT), which is defined as a regular, rapid rhythm that requires only atrial or atrioventricular tissue for its initiation and maintenance. Paroxysmal SVT (PSVT) is the most common dysrhythmia in children. It has an international prevalence of about 2 cases per 1,000 people. PSVT tends to manifest in infancy and early childhood.[4]\nFigure 1.\nThe presentation of PSVT widely varies in children, ranging from an incidental finding in an asymptomatic patient to fulminant cardiogenic shock. Infants present with caretakers complaining of rapid breathing, poor feeding, sweating with feeding, pallor, lethargy, and excessive crying. Older children may complain of chest pain, shortness of breath, and palpitations. The physical examination is likewise variable, depending upon the child's age and heart rate and on the duration of the episode (which can last from seconds to days). For infants, the examination is remarkable for a regular tachycardia. Normal resting heart rates for neonates can be up to 160 bpm. The upper limit of the normal heart rate decreases with age until late adolescence, when children's heart rates are similar to those of adults. Heart rates ranging beyond the normal upper limits, particularly in excess of 240, should be highly suspicious for SVT. In addition to tachycardia, infants may have physical findings of pallor, irritability, lethargy, tachypnea, weight loss (or failure to gain), poor perfusion, weak pulses/hypotension, hepatomegaly, and, sometimes, cardiogenic shock. A pounding sensation in the neck may be caused by cannon A waves, which occur when the atrium contracts at the same time as the ventricle. Older children typically have benign examinations (except for the findings of tachycardia and tachypnea).[1,3,6]\nThe 3 types of SVT are (1) atrial tachycardia (ectopic, or nonreciprocating, atrial tachycardia), (2) atrioventricular nodal reentrant tachycardia (AVNRT), and (3) atrioventricular reentrant (or reciprocating) tachycardia (AVRT). In the United States, reentrant tachycardias are the most common cause of PSVT in the pediatric population, AVRT is more common than AVRT in children younger than 12 years. AVRT consists of 2 or more functionally (and, usually, anatomically) distinct pathways between the atria and ventricles. The first pathway is usually the atrioventricular (AV) node. The second is an accessory pathway that may be an anatomically separate bypass tract between the atrium and ventricle (such as the bundle of Kent). Wolff-Parkinson-White (WPW) syndrome preexcitation is a good example of SVT caused by an anatomically separate bypass tract. Each pathway has different electrophysiologic characteristics; one pathway is fast (a short conduction time and a long refractory period), while the other is slow (a longer conduction time and a shorter refractory period). In AVNRT, both pathways exist in the AV node itself. In AVNRT, for example, while the child is in regular sinus, a premature atrial beat may block in the fast pathway (because of its longer refractory period). The classification of AVRT includes distinguishing antidromic (down the accessory pathway and back up the AV node) from orthodromic (down the AV node back up the accessory pathway).\nBecause the fast pathway is blocked, the signal conducts down the slow pathway. Then, when the impulse reaches the insertion of the fast pathway, which has now recovered after its refractory period, the impulse conducts in a retrograde fashion through the fast pathway. This results in a circuit loop tachycardia, with an impulse moving in a loop down the slow pathway and up the fast one.[1,3,4,5,6] A circuit loop in which impulses go down the slow pathway and back up the fast pathway is a typical AVNRT; in atypical AVNRT, the impulse goes down the fast pathway and back up the slow pathway. On an ECG these can be differentiated by where the P wave (atrial activation) is in relation to the QRS complex.\nOther causes of PSVT include sympathomimetic stimulation (such as medications for upper respiratory infection), structural defects, and atrial ectopy. About half of all SVT cases occur without underlying heart disease; these cases are termed idiopathic. Idiopathic SVT is more common in younger patients than in older children. WPW syndrome preexcitation accounts for 10-20% of cases. Congenital heart defects such as Ebstein anomaly may also predispose children to SVT. Children who have undergone cardiac surgery are also more prone to developing this arrhythmia.[3]\nThe diagnosis of PSVT is made based on patient history, physical examination findings, and ECG findings. The ECG findings include an excessively rapid (usually between 200 and 280 bpm) regular tachycardia, often without discernible P-waves preceding the QRS complexes. P-waves, when visible in PSVT, may have an abnormal axis and may be seen within or following the QRS complexes. In most cases, the tachycardia will be narrow complex; however, wide-complex PSVT is possible if aberrancy is present or if the AVNR conduction occurs in an antidromic fashion with WPW syndrome. It is important to distinguish PSVT from sinus tachycardia, which is the most common tachycardia in children. Sinus tachycardia and PSVT have dramatically different treatments. Laboratory evaluation depends on the clinical scenario, but practitioners should consider checking electrolytes, thyroid function, and hemoglobin level.[4]\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 3 of 5*",
"pagination": {
"current_page": 3,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 329839,
"choiceText": "Torsade de pointes",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329840,
"choiceText": "Atrial fibrillation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329841,
"choiceText": "Supraventricular tachycardia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329842,
"choiceText": "Ventricular tachycardia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 92180,
"questionText": "What type of arrhythmia is shown on the ECG?<br/>\r\n<br/>\r\n<em>Hint: Consider the morphology of QRS complexes (narrow vs wide) and the timing of the complexes (regular vs irregular).</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 8-Day-Old Boy With Tachypnea and Difficulty Feeding"
},
{
"authors": "Craig A. Goolsby, MD; Erik D. Schraga, MD",
"content": [
"Emergency management of children with PSVT should start with a focus on first assessing the airway for potential compromise or obstruction, then evaluating breathing and ventilation, and assessing the circulatory status of the patient (ie, the \"ABCs\"). When evaluating the circulatory status, unstable patients should undergo immediate synchronized cardioversion with 0.5 J/kg. The power should be increased as needed to 2 J/kg. If the cardioversion fails, overdrive pacing is another option. For stable patients, vagal maneuvers can be attempted first. Infants possess a diving reflex, in which vagal tone will increase in response to a cold stimulus (eg, ice) on their face. In older children, unilateral carotid massage, eyeball pressure, or even a headstand is more likely to cause conversion. When these maneuvers are not typically successful in converting the rhythm, medication is usually required. Adenosine, given at a dose of 0.1 mg/kg rapid intravenous push, is the first-line agent. This may be repeated in doses of 0.3 mg/kg, as needed. Amiodarone and procainamide are also safe in children with SVTs refractory to adenosine. In general, adenosine and digoxin should be avoided in situations where WPW syndrome is suspected, as they may potentiate conduction through the accessory pathway, resulting in 1:1 AV conduction increased ventricular rates and possible degeneration to ventricular fibrillation. Children without WPW syndrome preexcitation can be maintained on chronic oral propranolol or verapamil (in children older than 5 years), if needed after the acute illness. These agents should be used in consultation with a pediatric cardiologist.[1,2,3,4,5,6]",
"The disposition of children with PSVT depends on the clinical circumstance. A child in shock or with concerning comorbidities should be admitted to a pediatric intensive care unit (ICU). Asymptomatic children without frequent recurrence may be able to follow up with a cardiologist, without initiating medications. For younger infants, the admission threshold should be lower.[4]",
"The patient in this case failed cardioversion with vagal maneuvers but converted after 2 boluses of adenosine, which is consistent with a reentrant circuit involving the AV node. The patient was admitted to a monitored pediatric unit and evaluated by pediatric cardiology. The patient was initially discharged on digoxin; however, after further follow-up, the medication was discontinued, because the child's cardiologist came to suspect WPW syndrome as the likely etiology for the PSVT."
],
"date": "January 29, 2015",
"figures": [],
"markdown": "# An 8-Day-Old Boy With Tachypnea and Difficulty Feeding\n\n **Authors:** Craig A. Goolsby, MD; Erik D. Schraga, MD \n **Date:** January 29, 2015\n\n ## Content\n\n Emergency management of children with PSVT should start with a focus on first assessing the airway for potential compromise or obstruction, then evaluating breathing and ventilation, and assessing the circulatory status of the patient (ie, the \"ABCs\"). When evaluating the circulatory status, unstable patients should undergo immediate synchronized cardioversion with 0.5 J/kg. The power should be increased as needed to 2 J/kg. If the cardioversion fails, overdrive pacing is another option. For stable patients, vagal maneuvers can be attempted first. Infants possess a diving reflex, in which vagal tone will increase in response to a cold stimulus (eg, ice) on their face. In older children, unilateral carotid massage, eyeball pressure, or even a headstand is more likely to cause conversion. When these maneuvers are not typically successful in converting the rhythm, medication is usually required. Adenosine, given at a dose of 0.1 mg/kg rapid intravenous push, is the first-line agent. This may be repeated in doses of 0.3 mg/kg, as needed. Amiodarone and procainamide are also safe in children with SVTs refractory to adenosine. In general, adenosine and digoxin should be avoided in situations where WPW syndrome is suspected, as they may potentiate conduction through the accessory pathway, resulting in 1:1 AV conduction increased ventricular rates and possible degeneration to ventricular fibrillation. Children without WPW syndrome preexcitation can be maintained on chronic oral propranolol or verapamil (in children older than 5 years), if needed after the acute illness. These agents should be used in consultation with a pediatric cardiologist.[1,2,3,4,5,6]\nThe disposition of children with PSVT depends on the clinical circumstance. A child in shock or with concerning comorbidities should be admitted to a pediatric intensive care unit (ICU). Asymptomatic children without frequent recurrence may be able to follow up with a cardiologist, without initiating medications. For younger infants, the admission threshold should be lower.[4]\nThe patient in this case failed cardioversion with vagal maneuvers but converted after 2 boluses of adenosine, which is consistent with a reentrant circuit involving the AV node. The patient was admitted to a monitored pediatric unit and evaluated by pediatric cardiology. The patient was initially discharged on digoxin; however, after further follow-up, the medication was discontinued, because the child's cardiologist came to suspect WPW syndrome as the likely etiology for the PSVT.\n\n ## Figures\n\n \n*Page 4 of 5*",
"pagination": {
"current_page": 4,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 329843,
"choiceText": "Synchronized cardioversion with 0.5 J/kg",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329844,
"choiceText": "Adenosine",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329845,
"choiceText": "Digoxin",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329846,
"choiceText": "Propranolol",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329847,
"choiceText": "Verapamil",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Adenosine, given at a dose of 0.1 mg/kg rapid intravenous push, is the first-line agent for cardioversion of a stable patient with SVT. This may be repeated in doses of 0.3 mg/kg, as needed. Unstable patients with SVT should be treated immediately with electrical cardioversion at 0.5 J/kg. The power may be increased in increments of 0.5 J/kg, to a maximum of 2 J/kg. If cardioversion fails, overdrive pacing is another option. Digoxin, propranolol, and verapamil are all useful in preventing recurrence but are not typically recommended for cardioversion.<br><br>Adenosine and other direct AV nodal blockades are contraindicated if the SVT is thought to be caused by WPW because this would cause possible 1:1 AV conduction down the accessory pathway and higher ventricular rates.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 92181,
"questionText": "Which of the following medications is the recommended treatment option for cardioversion in a stable child with supraventricular tachycardia (SVT)?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 329848,
"choiceText": "Wolff-Parkinson-White (WPW) syndrome preexcitation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329849,
"choiceText": "Valvular disease",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329850,
"choiceText": "Medications",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329851,
"choiceText": "Idiopathic",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329852,
"choiceText": "Prior cardiac surgery",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although there are many potential causes for SVT (including WPW syndrome preexcitation, structural heart disease, prior cardiac surgery, and certain medications), about half of cases occur without underlying heart disease. These cases of idiopathic SVT are more common in younger patients than in older ones.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 92182,
"questionText": "What is the most common underlying cause for supraventricular tachycardia (SVT) in children?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 8-Day-Old Boy With Tachypnea and Difficulty Feeding"
},
{
"authors": "Craig A. Goolsby, MD; Erik D. Schraga, MD",
"content": [],
"date": "January 29, 2015",
"figures": [],
"markdown": "# An 8-Day-Old Boy With Tachypnea and Difficulty Feeding\n\n **Authors:** Craig A. Goolsby, MD; Erik D. Schraga, MD \n **Date:** January 29, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 5 of 5*",
"pagination": {
"current_page": 5,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 329843,
"choiceText": "Synchronized cardioversion with 0.5 J/kg",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329844,
"choiceText": "Adenosine",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329845,
"choiceText": "Digoxin",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329846,
"choiceText": "Propranolol",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329847,
"choiceText": "Verapamil",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Adenosine, given at a dose of 0.1 mg/kg rapid intravenous push, is the first-line agent for cardioversion of a stable patient with SVT. This may be repeated in doses of 0.3 mg/kg, as needed. Unstable patients with SVT should be treated immediately with electrical cardioversion at 0.5 J/kg. The power may be increased in increments of 0.5 J/kg, to a maximum of 2 J/kg. If cardioversion fails, overdrive pacing is another option. Digoxin, propranolol, and verapamil are all useful in preventing recurrence but are not typically recommended for cardioversion.<br><br>Adenosine and other direct AV nodal blockades are contraindicated if the SVT is thought to be caused by WPW because this would cause possible 1:1 AV conduction down the accessory pathway and higher ventricular rates.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 92181,
"questionText": "Which of the following medications is the recommended treatment option for cardioversion in a stable child with supraventricular tachycardia (SVT)?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 329848,
"choiceText": "Wolff-Parkinson-White (WPW) syndrome preexcitation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329849,
"choiceText": "Valvular disease",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329850,
"choiceText": "Medications",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329851,
"choiceText": "Idiopathic",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329852,
"choiceText": "Prior cardiac surgery",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although there are many potential causes for SVT (including WPW syndrome preexcitation, structural heart disease, prior cardiac surgery, and certain medications), about half of cases occur without underlying heart disease. These cases of idiopathic SVT are more common in younger patients than in older ones.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 92182,
"questionText": "What is the most common underlying cause for supraventricular tachycardia (SVT) in children?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 8-Day-Old Boy With Tachypnea and Difficulty Feeding"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 329839,
"choiceText": "Torsade de pointes",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329840,
"choiceText": "Atrial fibrillation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329841,
"choiceText": "Supraventricular tachycardia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329842,
"choiceText": "Ventricular tachycardia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 92180,
"questionText": "What type of arrhythmia is shown on the ECG?<br/>\r\n<br/>\r\n<em>Hint: Consider the morphology of QRS complexes (narrow vs wide) and the timing of the complexes (regular vs irregular).</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 329843,
"choiceText": "Synchronized cardioversion with 0.5 J/kg",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329844,
"choiceText": "Adenosine",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329845,
"choiceText": "Digoxin",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329846,
"choiceText": "Propranolol",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329847,
"choiceText": "Verapamil",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Adenosine, given at a dose of 0.1 mg/kg rapid intravenous push, is the first-line agent for cardioversion of a stable patient with SVT. This may be repeated in doses of 0.3 mg/kg, as needed. Unstable patients with SVT should be treated immediately with electrical cardioversion at 0.5 J/kg. The power may be increased in increments of 0.5 J/kg, to a maximum of 2 J/kg. If cardioversion fails, overdrive pacing is another option. Digoxin, propranolol, and verapamil are all useful in preventing recurrence but are not typically recommended for cardioversion.<br><br>Adenosine and other direct AV nodal blockades are contraindicated if the SVT is thought to be caused by WPW because this would cause possible 1:1 AV conduction down the accessory pathway and higher ventricular rates.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 92181,
"questionText": "Which of the following medications is the recommended treatment option for cardioversion in a stable child with supraventricular tachycardia (SVT)?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 329848,
"choiceText": "Wolff-Parkinson-White (WPW) syndrome preexcitation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329849,
"choiceText": "Valvular disease",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329850,
"choiceText": "Medications",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329851,
"choiceText": "Idiopathic",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 329852,
"choiceText": "Prior cardiac surgery",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although there are many potential causes for SVT (including WPW syndrome preexcitation, structural heart disease, prior cardiac surgery, and certain medications), about half of cases occur without underlying heart disease. These cases of idiopathic SVT are more common in younger patients than in older ones.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 92182,
"questionText": "What is the most common underlying cause for supraventricular tachycardia (SVT) in children?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
714326 | /viewarticle/714326 | [
{
"authors": "Faisal M. Mawri, MD; Nida Yousef, MD; Ammar Alhmood, MD; Walid Abuhammour, MD, FAAP, FIDSA",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 17-year-old male high school student presents to the pediatric infectious disease clinic with a 10-day history of a facial rash that is not improving (Figures 1-2).",
"Figure 1.",
"Figure 2.",
"The patient had previously visited his primary care provider (PCP), who started the patient on amoxicillin-clavulanic acid 8 days ago. The rash did not improve on the antibiotic, and as a result, it was discontinued and the patient switched to trimethoprim-sulfamethoxazole. No improvement was noted with the second round of antibiotic therapy; the rash continued to spread, and the lesions increased in number. The patient was subsequently advised to follow up with the infectious disease clinic.",
"At the infectious disease clinic, the patient states that the rash started with several pimples over the forehead and cheek and then continued to spread and involve most of the right side of his face. The lesions are not itchy, but they are painful. The patient has no known drug allergies. His immunizations are up to date. He is very active on the wrestling team and was happily preparing for an upcoming competition. The patient denies having any weight loss, headaches, dizziness, photophobia, fever, or chills. The family history is noncontributory."
],
"date": "July 09, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/714/326/714326-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/714/326/714326-thumb2.png"
}
],
"markdown": "# A Puzzling Facial Rash on a 17-Year-Old Boy\n\n **Authors:** Faisal M. Mawri, MD; Nida Yousef, MD; Ammar Alhmood, MD; Walid Abuhammour, MD, FAAP, FIDSA \n **Date:** July 09, 2018\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 17-year-old male high school student presents to the pediatric infectious disease clinic with a 10-day history of a facial rash that is not improving (Figures 1-2).\nFigure 1.\nFigure 2.\nThe patient had previously visited his primary care provider (PCP), who started the patient on amoxicillin-clavulanic acid 8 days ago. The rash did not improve on the antibiotic, and as a result, it was discontinued and the patient switched to trimethoprim-sulfamethoxazole. No improvement was noted with the second round of antibiotic therapy; the rash continued to spread, and the lesions increased in number. The patient was subsequently advised to follow up with the infectious disease clinic.\nAt the infectious disease clinic, the patient states that the rash started with several pimples over the forehead and cheek and then continued to spread and involve most of the right side of his face. The lesions are not itchy, but they are painful. The patient has no known drug allergies. His immunizations are up to date. He is very active on the wrestling team and was happily preparing for an upcoming competition. The patient denies having any weight loss, headaches, dizziness, photophobia, fever, or chills. The family history is noncontributory.\n\n ## Figures\n\n **Figure 1.** \n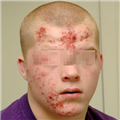 \n\n**Figure 2.** \n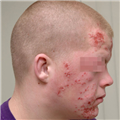 \n\n\n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 324329,
"choiceText": "Complete blood cell (CBC) count",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324330,
"choiceText": "Skin biopsy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324331,
"choiceText": "Enzyme-linked immunosorbent assay (ELISA)",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324332,
"choiceText": "Polymerase chain reaction (PCR)",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 90823,
"questionText": "Which of the following laboratory studies is likely to be most helpful in investigating this student's rash?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Puzzling Facial Rash on a 17-Year-Old Boy"
},
{
"authors": "Faisal M. Mawri, MD; Nida Yousef, MD; Ammar Alhmood, MD; Walid Abuhammour, MD, FAAP, FIDSA",
"content": [
"Upon physical examination, the patient is alert and orientated. His oral temperature is 97°F (36.1°C). The patient has normal heart sounds, his pulse has a regular rhythm of 97 beats/min, and his blood pressure is 125/75 mm Hg. His lungs are clear, and his respiratory rate is 12 breaths/min. The examination of the head, eyes, ears, and nose is remarkable for multiple vesicular lesions measuring about 0.5 cm in diameter (see Figures 1 and 2).",
"Figure 1.",
"Figure 2.",
"Bilateral submandibular lymph gland enlargement measures 1.5 × 1 cm. The neck is supple. His abdomen is soft and nontender to deep palpation in the epigastric region, and no organomegaly is noted. A CBC taken at the PCP's office showed a white blood cell (WBC) count of 7.4 × 103/µL (7.4 × 109/L), with a normal differential; a hemoglobin level of 13.6 g/dL (136 g/L); a hematocrit level of 38.3% (0.3830); and a platelet count of 298 × 103/uL (298 × 109/L)."
],
"date": "July 09, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/714/326/714326-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/714/326/714326-thumb2.png"
}
],
"markdown": "# A Puzzling Facial Rash on a 17-Year-Old Boy\n\n **Authors:** Faisal M. Mawri, MD; Nida Yousef, MD; Ammar Alhmood, MD; Walid Abuhammour, MD, FAAP, FIDSA \n **Date:** July 09, 2018\n\n ## Content\n\n Upon physical examination, the patient is alert and orientated. His oral temperature is 97°F (36.1°C). The patient has normal heart sounds, his pulse has a regular rhythm of 97 beats/min, and his blood pressure is 125/75 mm Hg. His lungs are clear, and his respiratory rate is 12 breaths/min. The examination of the head, eyes, ears, and nose is remarkable for multiple vesicular lesions measuring about 0.5 cm in diameter (see Figures 1 and 2).\nFigure 1.\nFigure 2.\nBilateral submandibular lymph gland enlargement measures 1.5 × 1 cm. The neck is supple. His abdomen is soft and nontender to deep palpation in the epigastric region, and no organomegaly is noted. A CBC taken at the PCP's office showed a white blood cell (WBC) count of 7.4 × 103/µL (7.4 × 109/L), with a normal differential; a hemoglobin level of 13.6 g/dL (136 g/L); a hematocrit level of 38.3% (0.3830); and a platelet count of 298 × 103/uL (298 × 109/L).\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 324329,
"choiceText": "Complete blood cell (CBC) count",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324330,
"choiceText": "Skin biopsy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324331,
"choiceText": "Enzyme-linked immunosorbent assay (ELISA)",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324332,
"choiceText": "Polymerase chain reaction (PCR)",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 90823,
"questionText": "Which of the following laboratory studies is likely to be most helpful in investigating this student's rash?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Puzzling Facial Rash on a 17-Year-Old Boy"
},
{
"authors": "Faisal M. Mawri, MD; Nida Yousef, MD; Ammar Alhmood, MD; Walid Abuhammour, MD, FAAP, FIDSA",
"content": [
"Herpes gladiatorum is an infection caused by the Herpes simplex virus (HSV) type 1. Herpes gladiatorum most commonly occurs among wrestlers and other athletes who participate in close skin contact sports such as wrestling (herpes gladiatorum) and rugby (herpes rugbiaforum). Herpes gladiatorum is also known as “mat herpes” among wrestlers. Herpes gladiatorum is spread by direct skin-to-skin contact. The lesions appear within 7 to 14 days after exposure on an infected person; however, in some cases the lesions take longer to appear.",
"The patient in this case presented with a primary herpes gladiatorum (PHG) infection, which is usually more severe than the recurrent infections. His lesions presented with disseminated vesicles, punched-out erosions, and central crusting on the forehead and right cheek. The patient was at an increased risk of contracting PHG because of his participation in wrestling. Herpes simplex virus DNA was detected with a polymerase chain reaction (PCR) examination.[1,2]",
"Outbreaks of herpes gladiatorum are common, particularly among wrestlers. Health care providers, athletic trainers, coaches, and athletes must recognize the threat of herpes gladiatorum skin infection to minimize the risk of outbreaks. Vigilant surveillance and appropriate antiviral treatment help curtail the transmission of herpes gladiatorum among wrestlers.[3] Lack of proper understanding of PHG disease can lead to misdiagnosis and frequent outbreaks.[4]"
],
"date": "July 09, 2018",
"figures": [],
"markdown": "# A Puzzling Facial Rash on a 17-Year-Old Boy\n\n **Authors:** Faisal M. Mawri, MD; Nida Yousef, MD; Ammar Alhmood, MD; Walid Abuhammour, MD, FAAP, FIDSA \n **Date:** July 09, 2018\n\n ## Content\n\n Herpes gladiatorum is an infection caused by the Herpes simplex virus (HSV) type 1. Herpes gladiatorum most commonly occurs among wrestlers and other athletes who participate in close skin contact sports such as wrestling (herpes gladiatorum) and rugby (herpes rugbiaforum). Herpes gladiatorum is also known as “mat herpes” among wrestlers. Herpes gladiatorum is spread by direct skin-to-skin contact. The lesions appear within 7 to 14 days after exposure on an infected person; however, in some cases the lesions take longer to appear.\nThe patient in this case presented with a primary herpes gladiatorum (PHG) infection, which is usually more severe than the recurrent infections. His lesions presented with disseminated vesicles, punched-out erosions, and central crusting on the forehead and right cheek. The patient was at an increased risk of contracting PHG because of his participation in wrestling. Herpes simplex virus DNA was detected with a polymerase chain reaction (PCR) examination.[1,2]\nOutbreaks of herpes gladiatorum are common, particularly among wrestlers. Health care providers, athletic trainers, coaches, and athletes must recognize the threat of herpes gladiatorum skin infection to minimize the risk of outbreaks. Vigilant surveillance and appropriate antiviral treatment help curtail the transmission of herpes gladiatorum among wrestlers.[3] Lack of proper understanding of PHG disease can lead to misdiagnosis and frequent outbreaks.[4]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 324333,
"choiceText": "Tinea faciale",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324334,
"choiceText": "Impetigo",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324335,
"choiceText": "Bacterial cellulitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324336,
"choiceText": "Herpes gladiatorum",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 90824,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Puzzling Facial Rash on a 17-Year-Old Boy"
},
{
"authors": "Faisal M. Mawri, MD; Nida Yousef, MD; Ammar Alhmood, MD; Walid Abuhammour, MD, FAAP, FIDSA",
"content": [
"HSV is a double-stranded DNA virus. About 80% of adults have antibodies to HSV-1, and about 20% of the population has antibodies to HSV-2. HSV-1 is commonly known to cause herpes labialis and keratitis. Most childhood herpes simplex virus infections are caused by HSV-1. HSV-2 is commonly known to cause genital herpes infections, which is one of the most common sexually transmitted diseases in the United States. HSV-2 is transmitted primarily by direct contact with lesions, and it is most often transmitted venereally.",
"Generalized or localized cutaneous and mucosal lesions characterize initial infection by HSV. Recurrent infections are milder because fewer viruses are shed and a stronger immune response is elicited. HSV remains dormant in the nerve ganglia; febrile illness, stress, immunosuppressive drugs, and ultraviolet light can precipitate recurrent eruptions. In rare cases, the initial replication of herpes simplex virus can lead to meningitis or encephalitis. HSV persists for life in a latent form. The virus is sometimes confused with herpes zoster because the site of latency for herpes simplex virus is the trigeminal ganglion. HSV may also manifest as a severe and/or life-threatening infection in immunocompromised individuals and in newborn babies. Specifically, disseminated infections can result in esophagitis, pneumonitis, encephalitis, hepatitis, and adrenal necrosis.[1,5]",
"The National Collegiate Athletic Association (NCAA) estimated an incidence of herpes gladiatorum as high as 40% among wrestlers.[6] The most common locations of herpes gladiatorum, in descending order, are the head, face, neck, chest, and shoulders. Typically, lesions are observed on the head, face, neck, cheeks, forehead, shoulders, and arms. According to most studies, about two thirds of wrestlers have the herpetic lesions on the right side of the body. Hence, herpes gladiatorum is transmitted during close skin-to-skin physical contact, known in wrestlers' terminology as the \"lock-up position.\"[2,5]",
"PHG generally presents with an erythematous rash, sore throat, fever, cervical lymphadenopathy, and vesicles. Occasionally, the herpes gladiatorum lesion lacks the grouped vesicles on an erythematous base, and it is sometimes mistaken for impetigo, acne, tinea corporis, atopic dermatitis, varicella, or scabies. A significant complication of PHG in wrestlers is dendritic keratitis with subsequent corneal scarring. Other ocular complications of PHG include conjunctivitis, scleritis, and uveitis.",
"Fluid from the base of unroofed vesicles can be sent for testing by PCR, the test of choice for diagnosing PHG. A Tzanck smear of scrapings from the base of vesicles demonstrates multinucleated giant cells, a finding that is highly indicative of herpes simplex virus infection."
],
"date": "July 09, 2018",
"figures": [],
"markdown": "# A Puzzling Facial Rash on a 17-Year-Old Boy\n\n **Authors:** Faisal M. Mawri, MD; Nida Yousef, MD; Ammar Alhmood, MD; Walid Abuhammour, MD, FAAP, FIDSA \n **Date:** July 09, 2018\n\n ## Content\n\n HSV is a double-stranded DNA virus. About 80% of adults have antibodies to HSV-1, and about 20% of the population has antibodies to HSV-2. HSV-1 is commonly known to cause herpes labialis and keratitis. Most childhood herpes simplex virus infections are caused by HSV-1. HSV-2 is commonly known to cause genital herpes infections, which is one of the most common sexually transmitted diseases in the United States. HSV-2 is transmitted primarily by direct contact with lesions, and it is most often transmitted venereally.\nGeneralized or localized cutaneous and mucosal lesions characterize initial infection by HSV. Recurrent infections are milder because fewer viruses are shed and a stronger immune response is elicited. HSV remains dormant in the nerve ganglia; febrile illness, stress, immunosuppressive drugs, and ultraviolet light can precipitate recurrent eruptions. In rare cases, the initial replication of herpes simplex virus can lead to meningitis or encephalitis. HSV persists for life in a latent form. The virus is sometimes confused with herpes zoster because the site of latency for herpes simplex virus is the trigeminal ganglion. HSV may also manifest as a severe and/or life-threatening infection in immunocompromised individuals and in newborn babies. Specifically, disseminated infections can result in esophagitis, pneumonitis, encephalitis, hepatitis, and adrenal necrosis.[1,5]\nThe National Collegiate Athletic Association (NCAA) estimated an incidence of herpes gladiatorum as high as 40% among wrestlers.[6] The most common locations of herpes gladiatorum, in descending order, are the head, face, neck, chest, and shoulders. Typically, lesions are observed on the head, face, neck, cheeks, forehead, shoulders, and arms. According to most studies, about two thirds of wrestlers have the herpetic lesions on the right side of the body. Hence, herpes gladiatorum is transmitted during close skin-to-skin physical contact, known in wrestlers' terminology as the \"lock-up position.\"[2,5]\nPHG generally presents with an erythematous rash, sore throat, fever, cervical lymphadenopathy, and vesicles. Occasionally, the herpes gladiatorum lesion lacks the grouped vesicles on an erythematous base, and it is sometimes mistaken for impetigo, acne, tinea corporis, atopic dermatitis, varicella, or scabies. A significant complication of PHG in wrestlers is dendritic keratitis with subsequent corneal scarring. Other ocular complications of PHG include conjunctivitis, scleritis, and uveitis.\nFluid from the base of unroofed vesicles can be sent for testing by PCR, the test of choice for diagnosing PHG. A Tzanck smear of scrapings from the base of vesicles demonstrates multinucleated giant cells, a finding that is highly indicative of herpes simplex virus infection.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A Puzzling Facial Rash on a 17-Year-Old Boy"
},
{
"authors": "Faisal M. Mawri, MD; Nida Yousef, MD; Ammar Alhmood, MD; Walid Abuhammour, MD, FAAP, FIDSA",
"content": [
"The NCAA has developed recommendations on “time until return to competition” for primary herpes gladiatorum and for recurrent infection. For a primary outbreak of herpes, the wrestler must be examined by a clinician or an experienced certified athletic trainer; the following recommendations must be met before a wrestler returns to competition[3]:",
"The athlete must have no signs of systemic symptoms of viral infection.",
"The athlete must be free of any new lesions for 3 days or more prior to the start of competition.",
"Skin lesions must be dry and surmounted by a firm adherent crust.",
"The wrestler must have been on appropriate antiviral therapy for at least 120 hours before the beginning of a competition.",
"For recurrent herpes gladiatorum, the NCAA has also established several recommendations. First, vesicles must be completely dry and crusted. Second, the wrestler must have been on appropriate dosage of antiviral therapy for 120 hours or more at the time of tournament. For questionable cases, a Tzanck preparation should be performed, and the wrestler's status should be deferred until Tzanck prep or herpes simplex virus assay results are available.[3]",
"Antiviral drugs with activity against viral DNA synthesis have been effective against PHG infections. Acyclovir, famciclovir, and valacyclovir inhibit virus replication and suppress clinical manifestations, but they are not a cure for PHG because herpes simplex virus remains latent in sensory ganglia. Oral acyclovir has been shown to be effective in suppressing PHG in wrestlers; it is the drug of choice for treating PHG. Acyclovir reduces the duration of symptomatic lesions and is indicated for patients presenting within 2-3 days of the appearance of a herpetic rash. Most patients on acyclovir experience less pain and quicker resolution of their vesicular lesions. Several effective treatments for adult patients include oral acyclovir 200 mg 5 times daily or 400 mg 3 times daily for 7-10 days or until clinical resolution occurs. The recommended dose of acyclovir for PHG in the pediatric age group is 20-30 mg/kg/d, in 5 divided doses, for 7-10 days. As with all infections, prevention is better than treatment.[6]",
"In addition to the above treatment, wrestlers must practice effective hygiene immediately after wrestling. They must frequently clean competition gear and change towels. Regular hand-washing and thorough cleaning of the mats is critical. Wrestling mats should be cleaned between matches with household bleach (one-quarter cup of bleach in 1 gallon of water). Early identification and treatment can allow the wrestler to return to participation earlier and prevent teammates from contracting the disease. Despite these precautions, PHG spreads during wrestling and other close-contact sports resulting from contact with asymptomatic infected athletes.[2,5]",
"The patient in this case was started on acyclovir 400 mg 3 times a day for 1 week at the first visit to the pediatric infectious disease clinic. His lesions were likely caused by PHG because the 2 courses of antibiotic treatment that had been initially tried by his primary care provider did not result in improvement. After 1 week of acyclovir, most of his facial lesions were dry and had an adherent crust; however, 2 lesions on his right shoulder remained moist but completely resolved by the second week of treatment. The patient and his parents were advised repeatedly to call and report any eye symptoms, seizure, altered mental status, personality changes, photophobia, or headaches.",
"On subsequent follow-up, the patient's 13-year-old brother had developed similar lesions; he had borrowed his older brother's headgear, which was the most likely the cause of his lesions. The rash on the brother also resolved after starting acyclovir."
],
"date": "July 09, 2018",
"figures": [],
"markdown": "# A Puzzling Facial Rash on a 17-Year-Old Boy\n\n **Authors:** Faisal M. Mawri, MD; Nida Yousef, MD; Ammar Alhmood, MD; Walid Abuhammour, MD, FAAP, FIDSA \n **Date:** July 09, 2018\n\n ## Content\n\n The NCAA has developed recommendations on “time until return to competition” for primary herpes gladiatorum and for recurrent infection. For a primary outbreak of herpes, the wrestler must be examined by a clinician or an experienced certified athletic trainer; the following recommendations must be met before a wrestler returns to competition[3]:\nThe athlete must have no signs of systemic symptoms of viral infection.\nThe athlete must be free of any new lesions for 3 days or more prior to the start of competition.\nSkin lesions must be dry and surmounted by a firm adherent crust.\nThe wrestler must have been on appropriate antiviral therapy for at least 120 hours before the beginning of a competition.\nFor recurrent herpes gladiatorum, the NCAA has also established several recommendations. First, vesicles must be completely dry and crusted. Second, the wrestler must have been on appropriate dosage of antiviral therapy for 120 hours or more at the time of tournament. For questionable cases, a Tzanck preparation should be performed, and the wrestler's status should be deferred until Tzanck prep or herpes simplex virus assay results are available.[3]\nAntiviral drugs with activity against viral DNA synthesis have been effective against PHG infections. Acyclovir, famciclovir, and valacyclovir inhibit virus replication and suppress clinical manifestations, but they are not a cure for PHG because herpes simplex virus remains latent in sensory ganglia. Oral acyclovir has been shown to be effective in suppressing PHG in wrestlers; it is the drug of choice for treating PHG. Acyclovir reduces the duration of symptomatic lesions and is indicated for patients presenting within 2-3 days of the appearance of a herpetic rash. Most patients on acyclovir experience less pain and quicker resolution of their vesicular lesions. Several effective treatments for adult patients include oral acyclovir 200 mg 5 times daily or 400 mg 3 times daily for 7-10 days or until clinical resolution occurs. The recommended dose of acyclovir for PHG in the pediatric age group is 20-30 mg/kg/d, in 5 divided doses, for 7-10 days. As with all infections, prevention is better than treatment.[6]\nIn addition to the above treatment, wrestlers must practice effective hygiene immediately after wrestling. They must frequently clean competition gear and change towels. Regular hand-washing and thorough cleaning of the mats is critical. Wrestling mats should be cleaned between matches with household bleach (one-quarter cup of bleach in 1 gallon of water). Early identification and treatment can allow the wrestler to return to participation earlier and prevent teammates from contracting the disease. Despite these precautions, PHG spreads during wrestling and other close-contact sports resulting from contact with asymptomatic infected athletes.[2,5]\nThe patient in this case was started on acyclovir 400 mg 3 times a day for 1 week at the first visit to the pediatric infectious disease clinic. His lesions were likely caused by PHG because the 2 courses of antibiotic treatment that had been initially tried by his primary care provider did not result in improvement. After 1 week of acyclovir, most of his facial lesions were dry and had an adherent crust; however, 2 lesions on his right shoulder remained moist but completely resolved by the second week of treatment. The patient and his parents were advised repeatedly to call and report any eye symptoms, seizure, altered mental status, personality changes, photophobia, or headaches.\nOn subsequent follow-up, the patient's 13-year-old brother had developed similar lesions; he had borrowed his older brother's headgear, which was the most likely the cause of his lesions. The rash on the brother also resolved after starting acyclovir.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 324341,
"choiceText": "Famciclovir",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324342,
"choiceText": "Valacyclovir",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324343,
"choiceText": "Acyclovir",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324344,
"choiceText": "Penciclovir",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324345,
"choiceText": "Ganciclovir",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Antiviral drugs with activity against viral DNA synthesis have been effective against PHG infections. Acyclovir, famciclovir, and valacyclovir inhibit virus replication and suppress clinical manifestations, but they are not a cure for PHG because herpes simplex virus remains latent in sensory ganglia. Oral acyclovir has been shown to be effective in suppressing PHG in wrestlers; it is the drug of choice for treating PHG. Acyclovir reduces the duration of symptomatic lesions and is indicated for patients presenting within 2-3 days of the appearance of a herpetic rash. Most patients on acyclovir experience less pain and quicker resolution of their vesicular lesions. Several effective treatments for adult patients include oral acyclovir 200 mg 5 times daily or 400 mg 3 times daily for 7-10 days or until clinical resolution occurs. The recommended dose of acyclovir for PHG in the pediatric age group is 20-30 mg/kg/d, in 5 divided doses, for 7-10 days. As with all infections, prevention is better than treatment.[6]",
"displayOrder": 4,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 90826,
"questionText": "Which of the following is the drug of choice for treating primary herpes gladiatorum?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 324346,
"choiceText": "The athlete must be free of systemic symptoms of viral infection before permitting participation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324347,
"choiceText": "The athlete must not have developed any new lesions 3 days before examination",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324348,
"choiceText": "All lesions must be dry and surmounted by a firm adherent crust",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324349,
"choiceText": "The athlete must be on appropriate antiviral therapy for at least 120 hours before competition",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324350,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In addition to all the criteria listed above, for recurrent herpes gladiatorum, the NCAA has also established several recommendations. First, vesicles must be completely dry and crusted. Second, the wrestler must have been on appropriate dosage of antiviral therapy for 120 hours or more at the time of tournament. For questionable cases, a Tzanck preparation should be performed, and the wrestler's status should be deferred until Tzanck prep or herpes simplex virus assay results are available.[3]",
"displayOrder": 5,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 90827,
"questionText": "Which of the following recommendations must be met before a wrestler can return to competition, according to the NCAA's \"time until return to competition\" guideline for primary herpes gladiatorum and for recurrent infection?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Puzzling Facial Rash on a 17-Year-Old Boy"
},
{
"authors": "Faisal M. Mawri, MD; Nida Yousef, MD; Ammar Alhmood, MD; Walid Abuhammour, MD, FAAP, FIDSA",
"content": [],
"date": "July 09, 2018",
"figures": [],
"markdown": "# A Puzzling Facial Rash on a 17-Year-Old Boy\n\n **Authors:** Faisal M. Mawri, MD; Nida Yousef, MD; Ammar Alhmood, MD; Walid Abuhammour, MD, FAAP, FIDSA \n **Date:** July 09, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 324341,
"choiceText": "Famciclovir",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324342,
"choiceText": "Valacyclovir",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324343,
"choiceText": "Acyclovir",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324344,
"choiceText": "Penciclovir",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324345,
"choiceText": "Ganciclovir",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Antiviral drugs with activity against viral DNA synthesis have been effective against PHG infections. Acyclovir, famciclovir, and valacyclovir inhibit virus replication and suppress clinical manifestations, but they are not a cure for PHG because herpes simplex virus remains latent in sensory ganglia. Oral acyclovir has been shown to be effective in suppressing PHG in wrestlers; it is the drug of choice for treating PHG. Acyclovir reduces the duration of symptomatic lesions and is indicated for patients presenting within 2-3 days of the appearance of a herpetic rash. Most patients on acyclovir experience less pain and quicker resolution of their vesicular lesions. Several effective treatments for adult patients include oral acyclovir 200 mg 5 times daily or 400 mg 3 times daily for 7-10 days or until clinical resolution occurs. The recommended dose of acyclovir for PHG in the pediatric age group is 20-30 mg/kg/d, in 5 divided doses, for 7-10 days. As with all infections, prevention is better than treatment.[6]",
"displayOrder": 4,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 90826,
"questionText": "Which of the following is the drug of choice for treating primary herpes gladiatorum?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 324346,
"choiceText": "The athlete must be free of systemic symptoms of viral infection before permitting participation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324347,
"choiceText": "The athlete must not have developed any new lesions 3 days before examination",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324348,
"choiceText": "All lesions must be dry and surmounted by a firm adherent crust",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324349,
"choiceText": "The athlete must be on appropriate antiviral therapy for at least 120 hours before competition",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324350,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In addition to all the criteria listed above, for recurrent herpes gladiatorum, the NCAA has also established several recommendations. First, vesicles must be completely dry and crusted. Second, the wrestler must have been on appropriate dosage of antiviral therapy for 120 hours or more at the time of tournament. For questionable cases, a Tzanck preparation should be performed, and the wrestler's status should be deferred until Tzanck prep or herpes simplex virus assay results are available.[3]",
"displayOrder": 5,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 90827,
"questionText": "Which of the following recommendations must be met before a wrestler can return to competition, according to the NCAA's \"time until return to competition\" guideline for primary herpes gladiatorum and for recurrent infection?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Puzzling Facial Rash on a 17-Year-Old Boy"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 324329,
"choiceText": "Complete blood cell (CBC) count",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324330,
"choiceText": "Skin biopsy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324331,
"choiceText": "Enzyme-linked immunosorbent assay (ELISA)",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324332,
"choiceText": "Polymerase chain reaction (PCR)",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 90823,
"questionText": "Which of the following laboratory studies is likely to be most helpful in investigating this student's rash?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 324333,
"choiceText": "Tinea faciale",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324334,
"choiceText": "Impetigo",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324335,
"choiceText": "Bacterial cellulitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324336,
"choiceText": "Herpes gladiatorum",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 90824,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 324341,
"choiceText": "Famciclovir",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324342,
"choiceText": "Valacyclovir",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324343,
"choiceText": "Acyclovir",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324344,
"choiceText": "Penciclovir",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324345,
"choiceText": "Ganciclovir",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Antiviral drugs with activity against viral DNA synthesis have been effective against PHG infections. Acyclovir, famciclovir, and valacyclovir inhibit virus replication and suppress clinical manifestations, but they are not a cure for PHG because herpes simplex virus remains latent in sensory ganglia. Oral acyclovir has been shown to be effective in suppressing PHG in wrestlers; it is the drug of choice for treating PHG. Acyclovir reduces the duration of symptomatic lesions and is indicated for patients presenting within 2-3 days of the appearance of a herpetic rash. Most patients on acyclovir experience less pain and quicker resolution of their vesicular lesions. Several effective treatments for adult patients include oral acyclovir 200 mg 5 times daily or 400 mg 3 times daily for 7-10 days or until clinical resolution occurs. The recommended dose of acyclovir for PHG in the pediatric age group is 20-30 mg/kg/d, in 5 divided doses, for 7-10 days. As with all infections, prevention is better than treatment.[6]",
"displayOrder": 4,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 90826,
"questionText": "Which of the following is the drug of choice for treating primary herpes gladiatorum?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 324346,
"choiceText": "The athlete must be free of systemic symptoms of viral infection before permitting participation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324347,
"choiceText": "The athlete must not have developed any new lesions 3 days before examination",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324348,
"choiceText": "All lesions must be dry and surmounted by a firm adherent crust",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324349,
"choiceText": "The athlete must be on appropriate antiviral therapy for at least 120 hours before competition",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324350,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In addition to all the criteria listed above, for recurrent herpes gladiatorum, the NCAA has also established several recommendations. First, vesicles must be completely dry and crusted. Second, the wrestler must have been on appropriate dosage of antiviral therapy for 120 hours or more at the time of tournament. For questionable cases, a Tzanck preparation should be performed, and the wrestler's status should be deferred until Tzanck prep or herpes simplex virus assay results are available.[3]",
"displayOrder": 5,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 90827,
"questionText": "Which of the following recommendations must be met before a wrestler can return to competition, according to the NCAA's \"time until return to competition\" guideline for primary herpes gladiatorum and for recurrent infection?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
714325 | /viewarticle/714325 | [
{
"authors": "Nicolai Wennike; Tim M. Battcock, MBChB, FRCP; Andrew J Bell, MA, MB, FRCP, FRCPath",
"content": [
"Editorial Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 53-year-old man who was diagnosed with multiple myeloma (IgAκ) 18 months ago is admitted to the hospital via the emergency department (ED) with a 1-week history of melena, hematemesis, and lethargy. He has no associated weight loss, abdominal pain, dysphagia, or history of upper gastrointestinal (GI) hemorrhage. The patient has no risk factors for peptic ulcer disease, does not drink alcohol or smoke, and is not regularly taking any medications (including no recent nonsteroidal anti-inflammatory drugs [NSAIDs] or steroid use).",
"He has no allergies of note, and his family history and social history are unremarkable. Other than multiple myeloma, which resulted in spinal cord compression that required radiotherapy (with full resolution of symptoms), the patient has no significant past medical history. He has not needed chemotherapy to date. On direct questioning, he does not describe any symptoms suggestive of active multiple myeloma and organ involvement."
],
"date": "April 27, 2018",
"figures": [],
"markdown": "# A 53-Year-Old Man With Melena and Hematemesis\n\n **Authors:** Nicolai Wennike; Tim M. Battcock, MBChB, FRCP; Andrew J Bell, MA, MB, FRCP, FRCPath \n **Date:** April 27, 2018\n\n ## Content\n\n Editorial Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 53-year-old man who was diagnosed with multiple myeloma (IgAκ) 18 months ago is admitted to the hospital via the emergency department (ED) with a 1-week history of melena, hematemesis, and lethargy. He has no associated weight loss, abdominal pain, dysphagia, or history of upper gastrointestinal (GI) hemorrhage. The patient has no risk factors for peptic ulcer disease, does not drink alcohol or smoke, and is not regularly taking any medications (including no recent nonsteroidal anti-inflammatory drugs [NSAIDs] or steroid use).\nHe has no allergies of note, and his family history and social history are unremarkable. Other than multiple myeloma, which resulted in spinal cord compression that required radiotherapy (with full resolution of symptoms), the patient has no significant past medical history. He has not needed chemotherapy to date. On direct questioning, he does not describe any symptoms suggestive of active multiple myeloma and organ involvement.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 53-Year-Old Man With Melena and Hematemesis"
},
{
"authors": "Nicolai Wennike; Tim M. Battcock, MBChB, FRCP; Andrew J Bell, MA, MB, FRCP, FRCPath",
"content": [
"Upon presentation, the patient appears clinically well, with no evidence of anemia, jaundice, lymphadenopathy, or peripheral signs of GI disease. He is hemodynamically stable, with a pulse of 90 beats/min, blood pressure of 150/70 mm Hg (with no postural blood pressure drop), and a urine output of approximately 30 mL/hr.",
"He has no evidence of active GI bleeding, his abdomen is soft and without any peritonitis or organomegaly, and a rectal examination shows evidence of melena, with no masses and a normal-sized prostate. His respiratory examination is unremarkable, with a clear chest and no evidence of aspiration pneumonia. The cardiac and neurologic examinations reveal nothing of significance.",
"The initial laboratory examinations show a hemoglobin level of 8.5 g/L (0.85 g/dL); a low mean corpuscular volume (79 fL), with an iron deficiency picture; a normal international normalized ratio of 1.0; and mild dehydration, with a urea nitrogen level of 10.1 mmol/L (28.29 mg/dL), creatinine level of 160 µmol/L (1.81 mg/dL), sodium level of 136 mmol/L (136 mEq/L), and potassium level of 3.9 mmol/L (3.9 mEq/L). Liver tests show a normal screen with an alanine aminotransferase level of 30 U/L, albumin level of 40 g/L (4 g/dL), alkaline phosphatase level of 50 U/L, and bilirubin level of 12 µmol/L (0.70 mg/dL). The patient is treated with intravenous fluid and 2 units of blood. He remains hemodynamically stable and is subsequently able to undergo an esophagogastroduodenoscopy (see Figure 1).",
"Figure 1."
],
"date": "April 27, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/714/325/714325-thumb1.png"
}
],
"markdown": "# A 53-Year-Old Man With Melena and Hematemesis\n\n **Authors:** Nicolai Wennike; Tim M. Battcock, MBChB, FRCP; Andrew J Bell, MA, MB, FRCP, FRCPath \n **Date:** April 27, 2018\n\n ## Content\n\n Upon presentation, the patient appears clinically well, with no evidence of anemia, jaundice, lymphadenopathy, or peripheral signs of GI disease. He is hemodynamically stable, with a pulse of 90 beats/min, blood pressure of 150/70 mm Hg (with no postural blood pressure drop), and a urine output of approximately 30 mL/hr.\nHe has no evidence of active GI bleeding, his abdomen is soft and without any peritonitis or organomegaly, and a rectal examination shows evidence of melena, with no masses and a normal-sized prostate. His respiratory examination is unremarkable, with a clear chest and no evidence of aspiration pneumonia. The cardiac and neurologic examinations reveal nothing of significance.\nThe initial laboratory examinations show a hemoglobin level of 8.5 g/L (0.85 g/dL); a low mean corpuscular volume (79 fL), with an iron deficiency picture; a normal international normalized ratio of 1.0; and mild dehydration, with a urea nitrogen level of 10.1 mmol/L (28.29 mg/dL), creatinine level of 160 µmol/L (1.81 mg/dL), sodium level of 136 mmol/L (136 mEq/L), and potassium level of 3.9 mmol/L (3.9 mEq/L). Liver tests show a normal screen with an alanine aminotransferase level of 30 U/L, albumin level of 40 g/L (4 g/dL), alkaline phosphatase level of 50 U/L, and bilirubin level of 12 µmol/L (0.70 mg/dL). The patient is treated with intravenous fluid and 2 units of blood. He remains hemodynamically stable and is subsequently able to undergo an esophagogastroduodenoscopy (see Figure 1).\nFigure 1.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 324276,
"choiceText": "Extraosseous spread of multiple myeloma",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324277,
"choiceText": "Gastric cancer",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324278,
"choiceText": "Peutz-Jeghers syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324279,
"choiceText": "Adenomatous polyps",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 90794,
"questionText": "What is the cause of the abnormalities seen on the endoscopy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 53-Year-Old Man With Melena and Hematemesis"
},
{
"authors": "Nicolai Wennike; Tim M. Battcock, MBChB, FRCP; Andrew J Bell, MA, MB, FRCP, FRCPath",
"content": [
"Biopsies of the polyps taken at the time of the endoscopy showed evidence of multiple myeloma type IgA κ. Multiple myeloma is a debilitating malignancy that is part of a spectrum of diseases ranging from monoclonal gammopathy of unknown significance (MGUS) to plasma cell leukemia. First documented in 1848, multiple myeloma is a disease characterized by a clonal proliferation of malignant B cells in the bone marrow (in which the predominant cell type is plasma cells) that results in an overabundance of monoclonal paraprotein. It is predominantly a disease of the elderly (median age: 60 years), with an incidence of 9.5 per 100,000 population and a slight male predominance. It is the second most common hematologic cancer (10%), representing 1% of all cases of cancer; despite new advances, multiple myeloma still carries a poor prognosis, with a median survival of 2-3 years.",
"The pathophysiology of multiple myeloma is that of a chromosomal translocation between the immunoglobulin heavy-chain gene (on the 14th chromosome, locus 14q32) and an oncogene (often 11q13, 4p16.3, 6p21, 16q23, or 20q11). This mutation results in dysregulation of the oncogene, which is thought to be an important initiating event in the pathogenesis of myeloma. The result of this mutation is proliferation of a plasma cell clone and genomic instability that leads to further mutations and translocations. The chromosome 14 abnormality is observed in about 50% of all myeloma cases; the other 50% of cases result from a deletion of (parts of) the 13th chromosome. The resulting plasma cells produce cytokines (especially interleukin [IL]–6) that cause osteopenia and create an environment for malignant cells to thrive."
],
"date": "April 27, 2018",
"figures": [],
"markdown": "# A 53-Year-Old Man With Melena and Hematemesis\n\n **Authors:** Nicolai Wennike; Tim M. Battcock, MBChB, FRCP; Andrew J Bell, MA, MB, FRCP, FRCPath \n **Date:** April 27, 2018\n\n ## Content\n\n Biopsies of the polyps taken at the time of the endoscopy showed evidence of multiple myeloma type IgA κ. Multiple myeloma is a debilitating malignancy that is part of a spectrum of diseases ranging from monoclonal gammopathy of unknown significance (MGUS) to plasma cell leukemia. First documented in 1848, multiple myeloma is a disease characterized by a clonal proliferation of malignant B cells in the bone marrow (in which the predominant cell type is plasma cells) that results in an overabundance of monoclonal paraprotein. It is predominantly a disease of the elderly (median age: 60 years), with an incidence of 9.5 per 100,000 population and a slight male predominance. It is the second most common hematologic cancer (10%), representing 1% of all cases of cancer; despite new advances, multiple myeloma still carries a poor prognosis, with a median survival of 2-3 years.\nThe pathophysiology of multiple myeloma is that of a chromosomal translocation between the immunoglobulin heavy-chain gene (on the 14th chromosome, locus 14q32) and an oncogene (often 11q13, 4p16.3, 6p21, 16q23, or 20q11). This mutation results in dysregulation of the oncogene, which is thought to be an important initiating event in the pathogenesis of myeloma. The result of this mutation is proliferation of a plasma cell clone and genomic instability that leads to further mutations and translocations. The chromosome 14 abnormality is observed in about 50% of all myeloma cases; the other 50% of cases result from a deletion of (parts of) the 13th chromosome. The resulting plasma cells produce cytokines (especially interleukin [IL]–6) that cause osteopenia and create an environment for malignant cells to thrive.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 324276,
"choiceText": "Extraosseous spread of multiple myeloma",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324277,
"choiceText": "Gastric cancer",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324278,
"choiceText": "Peutz-Jeghers syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324279,
"choiceText": "Adenomatous polyps",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 90794,
"questionText": "What is the cause of the abnormalities seen on the endoscopy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 53-Year-Old Man With Melena and Hematemesis"
},
{
"authors": "Nicolai Wennike; Tim M. Battcock, MBChB, FRCP; Andrew J Bell, MA, MB, FRCP, FRCPath",
"content": [
"The clinical manifestations of multiple myeloma vary because multiple organs can be involved. The clinical manifestations are related to substances secreted by the plasma cells and the effects of bone marrow infiltration; these effects can be classified into 3 categories:",
"Direct effects of plasma cell infiltration: The activation of osteoclasts by IL-6 causes bone destruction and lytic lesions, with resulting bone pain (especially headache); pathologic fractures and cord compression; symptomatic hypercalcemia, and osteopenia. The infiltration of bone marrow leads to pancytopenia, hypogammaglobulinemia, and paraproteinemia, which result in immunosuppression and susceptibility to pneumonias (especially Streptococcus pneumoniae and Staphylococcus aureus ) and Escherichia coli pyelonephritis.",
"Plasma protein abnormalities: The elevated production of paraproteins causes a hyperviscosity syndrome, with associated neurologic, renal, immunologic, and vascular complications.",
"Multifactorial: Extraosseous spread of multiple myeloma is mainly to the kidneys, although involvement of the GI tract (most frequently, the small intestines) has been occasionally reported. The immunoglobulin involved is mainly IgG; however, in this case, the subgroup was IgA. Renal failure is a multifactorial clinical manifestation that is caused by direct infiltration, hypercalcemia, hyperviscosity, glomerular deposition of amyloid, recurrent pyelonephritis, and light-chain precipitation in the renal tubules.",
"Investigations include a complete blood count (CBC) to evaluate for anemia, thrombocytopenia, or leukopenia; a comprehensive metabolic panel to assess a patient's total protein, albumin and globulin, blood urea nitrogen (BUN), creatinine, and uric acid); tests for elevated inflammatory markers (erythrocyte sedimentation rate; and tests for renal failure. The results of these tests should prompt an investigation for multiple myeloma using protein electrophoresis, with evidence of paraproteins and, occasionally, immune paresis, immunoglobulins, serum β2 microglobulin, and urine Bence-Jones protein. The workup for suspected multiple myeloma should then include a skeletal survey, a bone marrow biopsy, and immunohistochemistry, plus MRI scans, if cord compression is a concern.",
"Multiple myeloma can, in theory, produce all classes of immunoglobulin; IgG paraproteins are the most commonly produced, followed by IgA and IgM. IgD and IgE myeloma are very rare. In addition, light and/or heavy chains (the building blocks of antibodies) may be secreted in isolation: κ or λ light chains or any of the 5 types of heavy chains (α, γ, δ, ε, or μ heavy chains). Early involvement of hematologists should be sought for a possible bone marrow biopsy to estimate the percentage of bone marrow occupied by plasma cells. This percentage is used in the diagnostic criteria for myeloma. Bone marrow examination reveals plasma cell infiltration, often in sheets or clumps. The plasma cells are 2-3 times larger than typical lymphocytes, and they have eccentric nuclei that are smooth and round or oval in contour. They often have clumped chromatin, with a perinuclear halo or pale zone and basophilic cytoplasm. Immunohistochemistry is then used to detect plasma cells that express immunoglobulins in the cytoplasm but not usually on the surface; the specific markers for multiple myeloma are typically CD56, CD38, CD138 positive, CD19 negative, and CD45 negative."
],
"date": "April 27, 2018",
"figures": [],
"markdown": "# A 53-Year-Old Man With Melena and Hematemesis\n\n **Authors:** Nicolai Wennike; Tim M. Battcock, MBChB, FRCP; Andrew J Bell, MA, MB, FRCP, FRCPath \n **Date:** April 27, 2018\n\n ## Content\n\n The clinical manifestations of multiple myeloma vary because multiple organs can be involved. The clinical manifestations are related to substances secreted by the plasma cells and the effects of bone marrow infiltration; these effects can be classified into 3 categories:\nDirect effects of plasma cell infiltration: The activation of osteoclasts by IL-6 causes bone destruction and lytic lesions, with resulting bone pain (especially headache); pathologic fractures and cord compression; symptomatic hypercalcemia, and osteopenia. The infiltration of bone marrow leads to pancytopenia, hypogammaglobulinemia, and paraproteinemia, which result in immunosuppression and susceptibility to pneumonias (especially Streptococcus pneumoniae and Staphylococcus aureus ) and Escherichia coli pyelonephritis.\nPlasma protein abnormalities: The elevated production of paraproteins causes a hyperviscosity syndrome, with associated neurologic, renal, immunologic, and vascular complications.\nMultifactorial: Extraosseous spread of multiple myeloma is mainly to the kidneys, although involvement of the GI tract (most frequently, the small intestines) has been occasionally reported. The immunoglobulin involved is mainly IgG; however, in this case, the subgroup was IgA. Renal failure is a multifactorial clinical manifestation that is caused by direct infiltration, hypercalcemia, hyperviscosity, glomerular deposition of amyloid, recurrent pyelonephritis, and light-chain precipitation in the renal tubules.\nInvestigations include a complete blood count (CBC) to evaluate for anemia, thrombocytopenia, or leukopenia; a comprehensive metabolic panel to assess a patient's total protein, albumin and globulin, blood urea nitrogen (BUN), creatinine, and uric acid); tests for elevated inflammatory markers (erythrocyte sedimentation rate; and tests for renal failure. The results of these tests should prompt an investigation for multiple myeloma using protein electrophoresis, with evidence of paraproteins and, occasionally, immune paresis, immunoglobulins, serum β2 microglobulin, and urine Bence-Jones protein. The workup for suspected multiple myeloma should then include a skeletal survey, a bone marrow biopsy, and immunohistochemistry, plus MRI scans, if cord compression is a concern.\nMultiple myeloma can, in theory, produce all classes of immunoglobulin; IgG paraproteins are the most commonly produced, followed by IgA and IgM. IgD and IgE myeloma are very rare. In addition, light and/or heavy chains (the building blocks of antibodies) may be secreted in isolation: κ or λ light chains or any of the 5 types of heavy chains (α, γ, δ, ε, or μ heavy chains). Early involvement of hematologists should be sought for a possible bone marrow biopsy to estimate the percentage of bone marrow occupied by plasma cells. This percentage is used in the diagnostic criteria for myeloma. Bone marrow examination reveals plasma cell infiltration, often in sheets or clumps. The plasma cells are 2-3 times larger than typical lymphocytes, and they have eccentric nuclei that are smooth and round or oval in contour. They often have clumped chromatin, with a perinuclear halo or pale zone and basophilic cytoplasm. Immunohistochemistry is then used to detect plasma cells that express immunoglobulins in the cytoplasm but not usually on the surface; the specific markers for multiple myeloma are typically CD56, CD38, CD138 positive, CD19 negative, and CD45 negative.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 53-Year-Old Man With Melena and Hematemesis"
},
{
"authors": "Nicolai Wennike; Tim M. Battcock, MBChB, FRCP; Andrew J Bell, MA, MB, FRCP, FRCPath",
"content": [
"The diagnosis for symptomatic and asymptomatic multiple myeloma should be made in accordance with the International Myeloma Working Group criteria.[6]",
"Symptomatic myeloma is characterized by the following:",
"Clonal plasma cells >10% on bone marrow biopsy",
"Monoclonal protein in serum or urine",
"Evidence of end-organ damage, such as hypercalcemia, renal damage, anemia, frequent infections, amyloidosis, or hyperviscosity",
"Asymptomatic myeloma is characterized by the following:",
"Serum paraprotein >30 g/L and/or clonal plasma cells >10%",
"No myeloma-related organ impairment",
"The International Staging System for multiple myeloma is as follows[7]:",
"Stage 1 - β2microglubulin <3.5 mg/L; albumin >3.5 g/dL (mean survival, 62 months)",
"Stage 2 - β2microglubulin <3.5 mg/L; albumin <3.5 g/dL (mean survival, 45 months)",
"Stage 3 - β2microglubulin >5.5 mg/L; albumin <3.5 g/dL (mean survival, 29 months)",
"Management is focused on disease containment and suppression; it involves supportive therapy and treatment of complications. This includes pain control, treatment of fractures and cord compression with use of rehydration, loop diuretics, and bisphosphonates for hypercalcemia. A multidisciplinary team approach is used when there is any evidence of end-organ damage. Specific therapy includes combination chemotherapy (dexamethasone, thalidomide, cyclophosphamide, vincristine, and melphalan); if tolerated, high-dose chemotherapy and autologous stem cell transplantation can be used, although this does confer a 5-10% risk of mortality.",
"Despite the availability of current treatments, the natural history of multiple myeloma involves relapse following treatment. Depending on the patient's condition, the prior treatment modalities and time of relapse will guide the suitability of further chemotherapy and stem cell transplantation. The prognosis is still poor, however, and new cytogenetic analyses of myeloma cells are being investigated for prognostic value; the deletion of chromosome 13, nonhyperdiploidy, and the balanced translocations t(4;14) and t(14;16) confer a poorer prognosis. The cytogenetic abnormalities 11q13 and 6p21 are associated with a better prognosis.",
"This patient subsequently underwent melphalan chemotherapy and stem cell transplantation, and he made a full recovery. He did not have any further upper GI hemorrhages, and a repeat endoscopy showed resolution of his myeloma polyps (see Figures 1 and 2).",
"Figure 1.",
"Figure 2."
],
"date": "April 27, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/714/325/714325-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/714/325/714325-thumb2.png"
}
],
"markdown": "# A 53-Year-Old Man With Melena and Hematemesis\n\n **Authors:** Nicolai Wennike; Tim M. Battcock, MBChB, FRCP; Andrew J Bell, MA, MB, FRCP, FRCPath \n **Date:** April 27, 2018\n\n ## Content\n\n The diagnosis for symptomatic and asymptomatic multiple myeloma should be made in accordance with the International Myeloma Working Group criteria.[6]\nSymptomatic myeloma is characterized by the following:\nClonal plasma cells >10% on bone marrow biopsy\nMonoclonal protein in serum or urine\nEvidence of end-organ damage, such as hypercalcemia, renal damage, anemia, frequent infections, amyloidosis, or hyperviscosity\nAsymptomatic myeloma is characterized by the following:\nSerum paraprotein >30 g/L and/or clonal plasma cells >10%\nNo myeloma-related organ impairment\nThe International Staging System for multiple myeloma is as follows[7]:\nStage 1 - β2microglubulin <3.5 mg/L; albumin >3.5 g/dL (mean survival, 62 months)\nStage 2 - β2microglubulin <3.5 mg/L; albumin <3.5 g/dL (mean survival, 45 months)\nStage 3 - β2microglubulin >5.5 mg/L; albumin <3.5 g/dL (mean survival, 29 months)\nManagement is focused on disease containment and suppression; it involves supportive therapy and treatment of complications. This includes pain control, treatment of fractures and cord compression with use of rehydration, loop diuretics, and bisphosphonates for hypercalcemia. A multidisciplinary team approach is used when there is any evidence of end-organ damage. Specific therapy includes combination chemotherapy (dexamethasone, thalidomide, cyclophosphamide, vincristine, and melphalan); if tolerated, high-dose chemotherapy and autologous stem cell transplantation can be used, although this does confer a 5-10% risk of mortality.\nDespite the availability of current treatments, the natural history of multiple myeloma involves relapse following treatment. Depending on the patient's condition, the prior treatment modalities and time of relapse will guide the suitability of further chemotherapy and stem cell transplantation. The prognosis is still poor, however, and new cytogenetic analyses of myeloma cells are being investigated for prognostic value; the deletion of chromosome 13, nonhyperdiploidy, and the balanced translocations t(4;14) and t(14;16) confer a poorer prognosis. The cytogenetic abnormalities 11q13 and 6p21 are associated with a better prognosis.\nThis patient subsequently underwent melphalan chemotherapy and stem cell transplantation, and he made a full recovery. He did not have any further upper GI hemorrhages, and a repeat endoscopy showed resolution of his myeloma polyps (see Figures 1 and 2).\nFigure 1.\nFigure 2.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n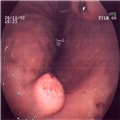 \n\n\n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 324284,
"choiceText": "Septicemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324285,
"choiceText": "Sickle cell anemia",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324286,
"choiceText": "Prolonged hypoxia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324287,
"choiceText": "Gaucher disease",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324288,
"choiceText": "Multiple myeloma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Unlike the other conditions, sickle cell anemia is not associated with a leukoerythroblastic anemia picture on blood film. Symptomatic myeloma is associated with end-organ damage, such as hypercalcemia, renal damage, anemia, frequent infections, amyloidosis, or hyperviscosity.",
"displayOrder": 4,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 90796,
"questionText": "Which of the following conditions are <i>not</i> associated with a leukoerythroblastic anemia picture on a blood film?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 324289,
"choiceText": "The incidence is higher in farmers and horticulturists.",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324290,
"choiceText": "The incidence increases in first-degree relatives.",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324291,
"choiceText": "The risk of developing multiple myeloma is higher in MGUS compared with solitary plasmacytoma.",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324292,
"choiceText": "High levels of β2 microglobulin is associated with a better prognosis.",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324293,
"choiceText": "The presence of plasma cells in a bone marrow biopsy confirms the diagnosis of multiple myeloma.",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Farmers have an increased risk for multiple myeloma. Individuals who have long-term exposure to pesticides typically have an increased multiple myeloma risk.",
"displayOrder": 5,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 90797,
"questionText": "Which of the following statements regarding multiple myeloma is true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 53-Year-Old Man With Melena and Hematemesis"
},
{
"authors": "Nicolai Wennike; Tim M. Battcock, MBChB, FRCP; Andrew J Bell, MA, MB, FRCP, FRCPath",
"content": [],
"date": "April 27, 2018",
"figures": [],
"markdown": "# A 53-Year-Old Man With Melena and Hematemesis\n\n **Authors:** Nicolai Wennike; Tim M. Battcock, MBChB, FRCP; Andrew J Bell, MA, MB, FRCP, FRCPath \n **Date:** April 27, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 324284,
"choiceText": "Septicemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324285,
"choiceText": "Sickle cell anemia",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324286,
"choiceText": "Prolonged hypoxia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324287,
"choiceText": "Gaucher disease",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324288,
"choiceText": "Multiple myeloma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Unlike the other conditions, sickle cell anemia is not associated with a leukoerythroblastic anemia picture on blood film. Symptomatic myeloma is associated with end-organ damage, such as hypercalcemia, renal damage, anemia, frequent infections, amyloidosis, or hyperviscosity.",
"displayOrder": 4,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 90796,
"questionText": "Which of the following conditions are <i>not</i> associated with a leukoerythroblastic anemia picture on a blood film?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 324289,
"choiceText": "The incidence is higher in farmers and horticulturists.",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324290,
"choiceText": "The incidence increases in first-degree relatives.",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324291,
"choiceText": "The risk of developing multiple myeloma is higher in MGUS compared with solitary plasmacytoma.",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324292,
"choiceText": "High levels of β2 microglobulin is associated with a better prognosis.",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324293,
"choiceText": "The presence of plasma cells in a bone marrow biopsy confirms the diagnosis of multiple myeloma.",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Farmers have an increased risk for multiple myeloma. Individuals who have long-term exposure to pesticides typically have an increased multiple myeloma risk.",
"displayOrder": 5,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 90797,
"questionText": "Which of the following statements regarding multiple myeloma is true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 53-Year-Old Man With Melena and Hematemesis"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 324276,
"choiceText": "Extraosseous spread of multiple myeloma",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324277,
"choiceText": "Gastric cancer",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324278,
"choiceText": "Peutz-Jeghers syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324279,
"choiceText": "Adenomatous polyps",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 90794,
"questionText": "What is the cause of the abnormalities seen on the endoscopy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 324284,
"choiceText": "Septicemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324285,
"choiceText": "Sickle cell anemia",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324286,
"choiceText": "Prolonged hypoxia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324287,
"choiceText": "Gaucher disease",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324288,
"choiceText": "Multiple myeloma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Unlike the other conditions, sickle cell anemia is not associated with a leukoerythroblastic anemia picture on blood film. Symptomatic myeloma is associated with end-organ damage, such as hypercalcemia, renal damage, anemia, frequent infections, amyloidosis, or hyperviscosity.",
"displayOrder": 4,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 90796,
"questionText": "Which of the following conditions are <i>not</i> associated with a leukoerythroblastic anemia picture on a blood film?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 324289,
"choiceText": "The incidence is higher in farmers and horticulturists.",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324290,
"choiceText": "The incidence increases in first-degree relatives.",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324291,
"choiceText": "The risk of developing multiple myeloma is higher in MGUS compared with solitary plasmacytoma.",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324292,
"choiceText": "High levels of β2 microglobulin is associated with a better prognosis.",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 324293,
"choiceText": "The presence of plasma cells in a bone marrow biopsy confirms the diagnosis of multiple myeloma.",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Farmers have an increased risk for multiple myeloma. Individuals who have long-term exposure to pesticides typically have an increased multiple myeloma risk.",
"displayOrder": 5,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 90797,
"questionText": "Which of the following statements regarding multiple myeloma is true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
Subsets and Splits
No community queries yet
The top public SQL queries from the community will appear here once available.